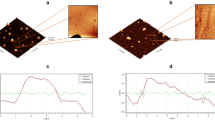
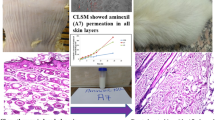
Abstract
The objective of the present work was to formulate a novel stable delivery system which would not only overcome the solubility issue of silymarin, but also help to increase the therapeutic value by better permeation, anticancer action and reduced toxicity. This was envisaged through the recent developments in nanotechnology, combined with the activity of the phytoconstituent silymarin. A 23 full factorial design based on three independent variables was used for process optimization of nanostructured lipid carriers (NLC). Developed formulations were evaluated on the basis of particle size, morphology, in vitro drug release, photostability and cell line studies. Optimized silymarin-NLC was incorporated into carbopol gel and further assessed for rheological parameters. Stable behaviour in presence of light was proven by photostability testing of formulation. Permeability parameters were significantly higher in NLC as compared to marketed phytosome formulation. The NLC based gel described in this study showed faster onset, and prolonged activity up to 24 h and better action against edema as compared to marketed formulation. In case of anticancer activity of silymarin-NLC against SK-MEL 2 cell lines, silymarin-NLC proved to possess anticancer activity in a dose-dependent manner (10–80 μM) and induced apoptosis at 80 μM in SK-MEL 2 cancer cells. This work documents for the first time that silymarin can be formulated into nanostructured lipoidal carrier system for enhanced permeation, greater stability as well as anticancer activity for skin.









Similar content being viewed by others
References
Lacatusu I, Mitrea E, Badea N, Stan R, Oprea O, Meghea A. Lipid nanoparticles based on omega-3 fatty acids as effective carriers for lutein delivery. Preparation and in vitro characterization studies. J Funct Foods. 2013;5(3):1260–9.
Kamble MS, Vaidya KK, Bhosale AV, Chaudhari PD. Solid lipid nanoparticles and nanostructured lipid carriers–an overview. Int J Pharma Chem Biol Sci. 2012;2(4):681–91.
Vitorino C, Almeida J, Gonçalves L, Almeida A, Sousa J, Pais A. Co-encapsulating nanostructured lipid carriers for transdermal application: from experimental design to the molecular detail. J Control Release. 2013;167(3):301–14.
Wissing S, Kayser O, Muller R. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56(9):1257–72.
Delgado D, Cruz R, Escobar-Chavez JJ, Ganem A. Preparation and characterization of triclosan nanoparticles intended to be used for treatment of acne. Eur J Pharm Biopharm. 2011;79(1):102–7.
Majumder J, Deb J, Das MR, Jana SS, Dastidar P. Designing a simple organic salt-based supramolecular topical gel capable of displaying in vivo self-delivery application. Chem Commun. 2014;50(14):1671–4.
Mei L, Zhang Y, Zheng Y, Tian G, Song C, Yang D, et al. A novel docetaxel-loaded poly (ε-caprolactone)/pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatment. Nanoscale Res Lett. 2009;4(12):1530–9.
Fang J-Y, Fang C-L, Liu C-H, Su Y-H. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2008;70(2):633–40.
Sanad RA, AbdelMalak NS, Badawi AA. Formulation of a novel oxybenzone-loaded nanostructured lipid carriers (NLCs). AAPS PharmSciTech. 2010;11(4):1684–94.
Teeranachaideekul V, Souto EB, Junyaprasert VB, Muller RH. Cetyl palmitate-based NLC for topical delivery of coenzyme Q (10)-development, physicochemical characterization and in vitro release studies. Eur J Pharm Biopharm. 2007;67(1):141–8.
Dong Y, Feng S-S. Poly (D, L-lactide-co-glycolide)(PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy. Int J Pharm. 2007;342(1):208–14.
W-H W, Lin B-Y, Kuo Y-H, Huang C-J. Triglycerides constituted of short and medium chain fatty acids and dicarboxylic acids in Momordicacharantia, as well as capric acid, inhibit PGE 2 production in RAW264. 7 macrophages. Food Chem. 2009;117(2):306–11.
Zhuang C-Y, Li N, Wang M, Zhang X-N, Pan W-S, Peng J-J, et al. Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm. 2010;394(1):179–85.
Chaturvedi SP, Kumar V. Production techniques of lipid nanoparticles: a review. RJPBCS. 2008;3(3):525–41.
Soumya D. A glimpse on melanoma-risk factors and treatment. J Cancer Sci Ther. 2011;17(4):1948–56.
Arias JL, Clares B, Morales ME, Gallardo V, Ruiz MA. Lipid-based drug delivery systems for cancer treatment. Curr Drug Targets. 2011;12(8):1151–65.
Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Cancer Nanotechnol: Methods Protocols. 2010;624:25–37.
Krena V, Walterova D. Silybin and silymarin-new effects and applications. Biomed Papers. 2005;149(1):29–41.
Spada G, Gavini E, Cossu M, Rassu G, Carta A, Giunchedi P. Evaluation of the effect of hydroxypropyl-β-cyclodextrin on topical administration of milk thistle extract. Carbohydr Polym. 2013;92(1):40–7.
Toklu HZ, Tunalı-Akbay T, Erkanlı G, Yuksel M, Ercan F, Şener G. Silymarin, the antioxidant component of Silybum marianum, protects against burn-induced oxidative skin injury. Burns. 2007;33(7):908–16.
Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89(8):556–65.
Katiyar SK. UV-induced immune suppression and photocarcinogenesis: chemoprevention by dietary botanical agents. Cancer Lett. 2007;255(1):1–11.
Singh RP, Agarwal R. Flavonoid antioxidant silymarin and skin cancer. Antioxid Redox Signal. 2002;4(4):655–63.
Munin A, Edwards-Levy F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics. 2011;3(4):793–829.
Parveen R, Baboota S, Ali J, Ahuja A, Vasudev SS, Ahmad S. Oil based nanocarrier for improved oral delivery of silymarin: in vitro and in vivo studies. Int J Pharm. 2011;413(1):245–53.
Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22(1):51–65.
Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev: J Clin Ther. 2011;16(3):239–49.
Rai R, Shanmuga SC, Srinivas C. Update on photoprotection. Indian J Dermatol. 2012;57(5):335.
Fang Z, Bhandari B. Encapsulation of polyphenols; a review. Trends Food Technol. 2010;21(10):510–23.
El-Sherbiny IM, Abdel-Mogib M, Dawidar A-AM, Elsayed A, Smyth HDC. Biodegradable pH-responsive alginate-poly (lactic-co-glycolic acid) nano/micro hydrogel matrices for oral delivery of silymarin. Carbohydr Polym. 2010;83(3):1345–54.
Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods. 2011;21(3):200–8.
Khan PA, Thube R, Rab RA. Formulation development and evaluation of silymarin gel for psoriasis treatment. J Innov Pharm Biol Sci. 2014;1(1):21–6.
Severino P, Santana MHA, Souto EB. Optimizing SLN and NLC by 2 2 full factorial design: effect of homogenization technique. Mater Sci Eng C. 2012;32(6):1375–9.
Teo BSX, Basri M, Zakaria MRS, Salleh AB, Rahman RN, Rahman MB. A potential tocopherol acetate loaded palm oil esters-in-water nanoemulsions for nanocosmeceuticals. J Nanobiotechnol. 2010;8(4):1–11.
Kumar R, Yasir M, Saraf SA, Gaur PK, Kumar Y, Singh AP. Glyceryl monostearate based nanoparticles of mefenamic acid: fabrication and in vitro characterization. Drug Invent Today. 2014;5(3):246–50.
Chawla V, Saraf SA. Glyceryl behenate and its suitability for production of aceclofenac solid lipid nanoparticles. J Am Oil Chem Soc. 2011;88(1):119–26.
Kamboj S, Saini V, Maggon N, Bala S, Jhawat V. Vesicular drug delivery systems: a novel approach for drug targeting. Int J Drug Deliv. 2013;5(2):121.
Mohamad NE, Abu N, Rahman HS, Ky H, Ho WY, Lim KL, et al. Nanostructured lipid carrier improved in vivo anti-tumor and immunomodulatory effect of Zerumbone in 4T1 challenged mice. RSC Adv. 2015;5(28):22066–74.
Shivhare UD, Jain KB, Mathur VB, Bhusari KP, Roy AA. Formulation development and evaluation of diclofenac sodium gel using water soluble polyacrylamide polymer. Dig J Nanomater Biostruct. 2009;4(2):285–90.
Patel D, Qasgupta S, Dey S, RojaRamani Y, Ray S, Mazumder B. Nanostructured lipid carriers (NLC)-based gel for topical delivery of aceclofenac: preparation, characterization and in vivo evaluation. Sci Pharm. 2012;80(3):749.
Zhao S, Yang X, Garamus VM, Handge UA, Berengere L, Zhao L, et al. Mixture of nonionic/ionic surfactants for the formulation of nanostructured lipid carriers: effects on physical properties. Langmuir. 2014;30(23):6920–8.
Tran TH, Ramasamy T, Truong DH, Choi H-G, Yong CS, Kim JO. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech. 2014;15(6):1509–15.
Misal G, Dixit G, Gulkari V. Formulation and evaluation of herbal gel. Indian J Nat Prod Res. 2012;3(4):501–5.
Gokce EH, Korkmaz E, Dellera E, Sandri G, Bonferoni MC, Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nanostructured lipid carriers: evaluation of antioxidant potential for dermal applications. Int J Nanomedicine. 2012;7(1):1841–50.
Agrawal Y, Petkar KC, Sawant KK. Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm. 2010;401(1):93–102.
Ravani L, Esposito E, Bories C, Lievin-Le Moal V, Loiseau PM, Djabourov M, et al. Clotrimazole-loaded nanostructured lipid carrier hydrogels: thermal analysis and in vitro studies. Int J Pharm. 2013;454(2):695–702.
Dosul J, Rodrigues O, Santos IR, Fillmann G, Matthiensen A. Skin irritation and histopathologic alterations in rats exposed to lightstick contents, UV radiation and seawater. Ecotoxicol Environ Saf. 2009;72(7):2020–4.
Majumder J, Yedoti P, Dastidar P. A supramolecular topical gel derived from a non-steroidal anti-inflammatory drug, fenoprofen, is capable of treating skin inflammation in mice. Org Biomol Chem. 2015;13(8):2300–9.
Ioele G, Cione E, Risoli A, Genchi G, Ragno G. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int J Pharm. 2005;293(1):251–60.
Joshi M, Patravale V. Nanostructured lipid carrier (NLC) based gel of celecoxib. Int J Pharm. 2008;346(1):124–32.
Pokharkar VB, Shekhawat PB, Dhapte VV, Mandpe LP. Development and optimization of eugenol loaded nanostructured lipid carriers for periodontal delivery Int J Pharm Pharmsci. 2011;3(4):138–143.
Wu N, Wang L-S, Tan DC-W, Moochhala SM, Yang Y-Y. Mathematical modeling and in vitro study of controlled drug release via a highly swellable and dissoluble polymer matrix: polyethylene oxide with high molecular weights. J Control Release. 2005;102(3):569–81.
Anthony FA, Dowdy JC, Costlow ME. Attenuation of ultraviolet radiation-induced edema and erythema with topical calmodulin and protein kinase C inhibitors. Photodermatol Photoimmunol Photomed. 1994;10(6):227–34.
Xia WJ, Onyuksel H. Mechanistic studies on surfactant-induced membrane permeability enhancement. Pharm Res. 2000;17(5):612–8.
Moribe K, Limwikrant W, Higashi K, Yamamoto K. Drug nanoparticle formulation using ascorbic acid derivatives. J Drug Deliv. 2011;2011(2011):9.
Learn DB, Beasley DG, Giddens LD, Beard J, Stanfield JW, Roberts LK. Minimum doses of ultraviolet radiation required to induce murine skin edema and immunosuppression are different and depend on the ultraviolet emission spectrum of the source. Photochem Photobiol. 1995;62(6):1066–75.
Mitchell DL, Byrom M, Chiarello S, Lowery MG. Effects of chronic exposure to ultraviolet B radiation on DNA repair in the dermis and epidermis of the hairless mouse. J Investig Dermatol. 2001;116(2):209–15.
McGlade JP, Gorman S, Lenzo JC, Tan JW, Watanabe T, Finlay-Jones JJ, et al. Effect of both ultraviolet B irradiation and histamine receptor function on allergic responses to an inhaled antigen. J Immunol. 2007;178(5):2794–802.
Inomata S, Matsunaga Y, Amano S, Takada K, Kobayashi K, Tsunenaga M, et al. Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J Investig Dermatol. 2003;120(1):128–34.
Kovacevic A, Savic S, Vuleta G, Muller RH, Keck CM. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): effects on size, physical stability and particle matrix structure. Int J Pharm. 2011;406(1):163–72.
Uprit S, Sahu RK, Roy A, Pare A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm J. 2013;21(4):379–85.
Joshi M, Patravale V. Formulation and evaluation of nanostructured lipid carrier (NLC) based gel of valdecoxib. Drug Dev Ind Pharm. 2006;32(8):911–8.
Galindo-Rodriguez S, Allamann E, Fessi H, Doelker E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm Res. 2004;21(8):1428–39.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 1). Trop J Pharm Res. 2013;12(2):255–64.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (part 2). Trop J Pharm Res. 2013;12(2):265–73.
Tantra R, Schulze P, Quincey P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology. 2010;8(3):279–85.
Basson DK, Berres S, Bürger R. On models of polydisperse sedimentation with particle-size-specific hindered-settling factors. Appl Math Model. 2009;33(4):1815–35.
Katiyar SK. Silymarin and skin cancer prevention: anti-inflammatory, antioxidant and immunomodulatory effects (review). Int J Oncol. 2005;26(1):169–76.
Lahiri-Chatterjee M, Katiyar SK, Mohan RR, Agarwal R. A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res. 1999;59(3):622–32.
Svobodova A, Zdarilova A, Maliskova J, Mikulkova H, Walterova D, Vostalova J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J Dermatol Sci. 2007;46(1):21–30.
Kuete V, Voukeng IK, Tsobou R, Mbaveng AT, Wiench B, Beng VP, et al. Cytotoxicity of Elaoephorbia drupifera and other Cameroonian medicinal plants against drug sensitive and multidrug resistant cancer cells. BMC Complement Altern Med. 2013;13(1):1.
Akindele AJ, Wani ZA, Sharma S, Mahajan G, Satti NK, Adeyemi OO, et al. In vitro and in vivo anticancer activity of root extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evid Based Complement Alternat Med. 2015;2015.
Acknowledgment
The authors wish to acknowledge Alchem Int. Pvt. Ltd., India and Gattefosse, India for the gift samples of excipients and drug ACTREC, Mumbai, India for some facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol for the experimentation, transportation and care of the animals used in study was approved by Institutional Animal Ethical Committee (BBDNIIT/IAEC/057/2014) and the handling was done as per CPCSEA guidelines.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
(DOCX 20 kb)
Supplementary Figure 2
(DOCX 236 kb)
Rights and permissions
About this article
Cite this article
Singh, P., Singh, M., Kanoujia, J. et al. Process optimization and photostability of silymarin nanostructured lipid carriers: effect on UV-irradiated rat skin and SK-MEL 2 cell line. Drug Deliv. and Transl. Res. 6, 597–609 (2016). https://doi.org/10.1007/s13346-016-0317-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0317-8