Abstract
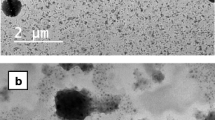
Ophthalmic nanosuspensions (ONS) have shown a potential for ophthalmic delivery over the conventional ocular formulations. The objective of the study was to assess the effect of surfactants and polymers on particle size and drug release. Sparfloxacin ONS were prepared by optimizing the concentration of HPMC E5 and water soluble chitosan by using solvent diffusion method followed by probe sonication. The Poloxamer 407 and Kolliphor P188 were used as a surfactant. The produced nanosuspensions were characterized for particle size, shape, zeta potential and drug release. The average particle size of the nanosuspension was 300 to 500 nm. The in vitro drug release study showed that the optimized nanosuspension of water soluble chitosan sustained drug release up to 9 h compared to 6 h for the hydroxypropylmethylcellulose (HPMC) nanosuspension. Further, the sparfloxacin ONS formulation showed excellent ocular tolerance and biocompatibility as determined by hen’s egg test chorioallantoic membrane (HET CAM) and resazurin assay on Vero cell lines. Moreover, optimized formulation was found to be stable, isotonic, non-toxic with higher in vitro and in vivo antimicrobial potential.






Similar content being viewed by others
References
Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310:1721–29.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A. Nanoparticles laden in situ gel for sustained ocular drug delivery. J Pharm Bioall Sci. 2013;5(2):162–5.
Takamura E, Uchio E, Ebihara N, Ohno S, Ohashi Y, Okamoto S, et al. Japanese guideline for allergic conjunctival diseases. Allergol Int. 2011;60:191–203.
Khimdas S, Visscher KL, Hutnik CML. Besifloxacin ophthalmic suspension : emerging evidence of its therapeutic value in bacterial conjunctivitis. Ophthalmol Eye Dis 2011; 37–42.
O’Brien TP, Thanathanee O Refining treatment of bacterial conjunctivitis. Ophthalmol Manag 2011; 4 [Online] http://www.ophthalmologymanagement.com/printarticle.aspx?articleID=105514.
Gawad AE, Gawad HAE, Soliman OA, Barker SA, Girgis GNS. Formulation and physical characterization of a novel sustained-release ophthalmic delivery system for sparfloxacin : the effect of the biological environment. Ophthalmol Res Int J. 2013;1(1):1–22.
Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56(7):827–40.
Das S, Suresh PK. Nanosuspension : a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to amphotericin B. Nanomed Nanotechnol Biol Med. 2011;7(2):242–47.
Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug nanocrystals : in vivo performances. J Control Release. 2012;160(3):418–30.
Reddy JS, Ahmed MG. Sustained ocular delivery of sparfloxacin from pH triggered in situ gelling system. MU J Pharm. 2013;40(3):16–25.
Talekar M, Kendall J, Denny W, Jamieson S, Garg S. European journal of pharmaceutical sciences development and evaluation of PIK75 nanosuspension, a phosphatidylinositol-3-kinase inhibitor. Eur J Pharm Sci. 2012;47(5):824–33.
Khadka P, Ro J, Kim H, Lee H. Pharmaceutical particle technologies: an approach to improve drug solubility, dissolution and bioavailability. AJPS. 2014;9(6):304–16.
Liu L, Lu Y. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013: 1921–1933.
Kim D, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27:3031–7.
Fan L, Du Y, Zhang B, Yang J. Preparation and properties of alginate/carboxymethyl chitosan blend fibers. Carbohydr Polym. 2006;65:447–52.
Anitha A, Rani VVD, Krishna R, Sreeja V, Selvamurugan N, Nair SV. Synthesis, characterization, cytotoxicity and antibacterial studies of chitosan, O -carboxymethyl and N, O -carboxymethyl chitosan nanoparticles. Carbohydr Polym. 2009;78(4):672–77.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–41.
Dandagi P, Kerur S, Mastiholimath V. Polymeric ocular nanosuspension for controlled release of acyclovir : in vitro release and ocular distribution. IJPR. 2009;8:79–86.
Mourya VK, Inamdar NN, Tiwari A. Carboxymethyl chitosan and its applications. Adv Mat Lett. 2010;1(1):11–33.
Upadhyaya L, Singh J, Agarwal V, Prakash R. Biomedical applications of carboxymethyl chitosans. Carbohydr Polym. 2013;91(1):452–66.
Ali HSM, York P, Ali MA, Blagden N. Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release. 2011;149:175–81.
Ige PP, Baria RK, Gattani SG. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Collo Surf Biointer. 2013;108:366–73.
Shinde U, Ahmed MH, Singh K. Development of dorzolamide loaded 6-O -carboxymethyl chitosan nanoparticles for open angle glaucoma. JDD 2013:1–15.
Dilbaghi N, Kaur H, Ahuja M, Kumar S. Evaluation of tropicamide-loaded tamarind seed xyloglucan nanoaggregates for ophthalmic delivery. Carbohydr Polym. 2013;94:286–91.
Gupta H, Aqil M, Khar RK, Ali A, Bhatnagar A, Mittal G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine. 2010;6(2):324–33.
Wu L, Zhang J, Watanable W. Physical and chemical stability of drug nanoparticles. Adv Drug Deliv Rev. 2011;63:456–69.
Patel CJ, Patel JV. Formulation and evaluation of chitosan nanoparticles containing glibenclamide. IJPRS. 2014;3:58–68.
Subedi RK, Kang KW, Choi HK. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009;37:508–13.
Fang JY, Fang CL, Liu CH, Su YH. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm. 2008;70:633–40.
Kongsong M, Songsurang K, Sangvanich P, Siralertmukul K. Design, synthesis, fabrication and in vitro evalution of mucoadhesive 5-amino-2-mercaptobenzimidazole chitosan as low water soluble drug carriers. Eur J Pharm Biopharm. 2014;88:986–97.
Sutradhar KB, Khatun S, Luna IP. Increasing possibilities of nanosuspension. JNT 2013; http://dx.doi.org/10.1155/2013/346581.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement of human and animal rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study. All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 206 kb)
Rights and permissions
About this article
Cite this article
Ambhore, N.P., Dandagi, P.M. & Gadad, A.P. Formulation and comparative evaluation of HPMC and water soluble chitosan-based sparfloxacin nanosuspension for ophthalmic delivery. Drug Deliv. and Transl. Res. 6, 48–56 (2016). https://doi.org/10.1007/s13346-015-0262-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0262-y