Abstract
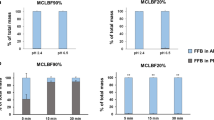
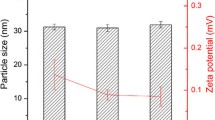
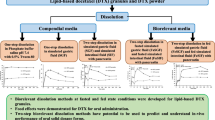
Lipid-based liquid crystalline (LC) systems have the potential to sustain the oral absorption of poorly water-soluble drugs in vivo, facilitating slow drug release from their complex internal structure. To further evaluate the dynamic relationship between gastric retention and sustained drug absorption for these systems, this study aimed to explore non-invasive X-ray micro-CT imaging as an approach to assess gastric retention. Pharmacokinetic studies were also conducted with cinnarizine-loaded LC formulations to correlate gastric retention of the formulation to drug absorption. The in vivo studies demonstrated the interplay between gastric retention and drug absorption based on the digestibility of the LC structures. An increase in non-digestible phytantriol (PHY) composition in the formulation relative to digestible glyceryl monooleate (GMO) increased the gastric retention, with 68 ± 4 % of formulation intensity remaining at 8 h for 85 % w/w PHY, and 26 ± 9 % for 60 % w/w PHY. Interestingly, it was found that PHY 30 % w/w in GMO provided the highest bioavailability for cinnarizine (CZ) amongst the other combinations, including GMO alone. The studies demonstrated that combining digestible and non-digestible lipids into LC systems allowed for an optimal balance between sustaining drug absorption whilst increasing plasma concentration (C max) over time, leading to enhanced oral bioavailability. The results demonstrate the potential for utilising non-invasive X-ray micro-CT imaging to dynamically assess the GI transit of orally administered liquid crystal-forming formulations.









Similar content being viewed by others
References
Nguyen T-H, Hanley T, Porter CJH, Boyd BJ. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J Control Release. 2011;153:180–6. Elsevier B.V.
Nguyen T-H, Hanley T, Porter CJH, Larson I, Boyd BJ. Phytantriol and glyceryl monooleate cubic liquid crystalline phases as sustained-release oral drug delivery systems for poorly water-soluble drugs II. In-vivo evaluation. J Pharm Pharmacol. 2010;62:856–65.
Boyd BJ, Khoo S-M, Whittaker DV, Davey G, Porter CJH. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int J Pharm. 2007;340:52–60.
Nielsen LS, Schubert L, Hansen J. Bioadhesive drug delivery systems. I. Characterisation of mucoadhesive properties of systems based on glyceryl mono-oleate and glyceryl monolinoleate. Eur J Pharm Sci. 1998;6:231–9.
Du JD, Liu Q, Salentinig S, Nguyen T-H, Boyd BJ. A novel approach to enhance the mucoadhesion of lipid drug nanocarriers for improved drug delivery to the buccal mucosa. Int J Pharm. 2014;471:358–65.
Tokumura T, Tsushima Y, Tatsuishi K, Kayano M, Machida Y, Nagai T. Enhancement of the oral bioavailability of cinnarizine in oleic acid in beagle dogs. J Pharm Sci. 1987;76:286–8.
Lee KWY, Porter CJH, Boyd BJ. A simple quantitative approach for the determination of long and medium chain lipids in bio-relevant matrices by high performance liquid chromatography with refractive index detection. AAPS PharmSciTech. 2013;14:927–34.
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–7.
Jackson PA, Rahman WNWA, Wong CJ, Ackerly T, Geso M. Potential dependent superiority of gold nanoparticles in comparison to iodinated contrast agents. Eur J Radiol. 2010;75:104–9. Elsevier Ireland Ltd.
Clogston J, Rathman J, Tomasko D, Walker H, Caffrey M. Phase behavior of a monoacylglycerol: (myverol 18-99K)/water system. Chem Phys Lipids. 2000;107:191–220.
Kirby NM, Mudie ST, Hawley AM, Cookson DJ, Mertens HDT, Cowieson N, et al. A low-background-intensity focusing small-angle X-ray scattering undulator beamline. J Appl Crystallogr. 2013;46:1670–80. International Union of Crystallography.
Hyde ST. Identification of lyotropic liquid crystalline mesophases. In: Holmberg K, editor. Handbook applied surface and colloid chemistry. New York: John Wiley & Sons, Ltd; 2001. p. 299–332.
Du JD, Fong W-K, Salentinig S, Caliph SM, Hawley A, Boyd BJ. Phospholipid-based self-assembled mesophase systems for light-activated drug delivery. Phys Chem Chem Phys. 2015;17:14021–7. Royal Society of Chemistry.
Fong W-K, Hanley TL, Thierry B, Kirby N, Waddington LJ, Boyd BJ. Controlling the nanostructure of gold nanorod-lyotropic liquid-crystalline hybrid materials using near-infrared laser irradiation. Langmuir. 2012;28:14450–60.
Bhosale RR, Osmani RA, Harkare BR, Ghodake PP. Review article cubosomes: the inimitable nanoparticulate drug carriers. Sch Acad J Pharm. 2013;2:481–6.
Doweck I, Gordon CR, Spitzer O, Melamed Y, Shupak A. Effect of cinnarizine in the prevention of seasickness. Aviat Space Environ Med. 1994;65:606–9.
Arab SF, Düwel P, Jüngling E, Westhofen M, Lückhoff A. Inhibition of voltage-gated calcium currents in type II vestibular hair cells by cinnarizine. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:570–5.
Nowacka-Krukowska H, Rakowska M, Neubart K, Kobylińska M. High-performance liquid chromatographic assay for cinnarizine in human plasma. Acta Pol Pharm. 2007;64:407–11.
Nguyen T-H, Hanley T, Porter CJH, Boyd BJ. Nanostructured reverse hexagonal liquid crystals sustain plasma concentrations for a poorly water-soluble drug after oral administration. Drug Deliv Transl Res. 2011;1:429–38.
Acknowledgments
Funding is acknowledged from the Australian Research Council under the Discovery Projects scheme DP120104032. BJB is a recipient of an Australian Research Council Future Fellowship (FT120100697). We acknowledge Tanmay Joshi and Olga Gazukin for transmission electron microscopy (TEM) images, Bim Graham and Raymond Lam for GNP synthesis, Joanne Du and Linda Hong for SAXS analysis and Orlagh Feeney for assistance with UPLC. We also acknowledge the MBI Facility for provision of instrumentation, training and general support. SAXS measurements were performed on the SAXS/WAXS beamline at the Australian Synchrotron facility.
Compliance with ethical standards
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1315 kb)
Rights and permissions
About this article
Cite this article
Pham, A.C., Nguyen, TH., Nowell, C.J. et al. Examining the gastrointestinal transit of lipid-based liquid crystalline systems using whole-animal imaging. Drug Deliv. and Transl. Res. 5, 566–574 (2015). https://doi.org/10.1007/s13346-015-0253-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0253-z