Abstract
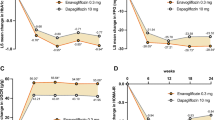
STELLA-LONG TERM, a 3-year post-marketing surveillance study, evaluated the safety and effectiveness of the sodium-glucose cotransporter 2 inhibitor ipragliflozin in Japanese type 2 diabetes mellitus (T2DM) patients. Final results in the safety (n = 6697) and effectiveness populations (n = 5625) were analyzed by stratifying patients by baseline estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) into four subgroups (≥ 90, 60 to < 90, 45 to < 60, and < 45) and two subgroups (≥ 60 and < 60). Adverse drug reaction (ADR) incidence, and changes from baseline in glycosylated hemoglobin (HbA1c), bodyweight, and eGFR were assessed. The percentage of patients experiencing ADRs and serious ADRs was similar across most eGFR subgroups. Polyuria/pollakiuria was the most common ADR. Renal disorders and volume depletion ADRs were more frequent in the subgroups with more severe renal impairment at baseline than in those with an eGFR of 60 to < 90 or ≥ 90 mL/min/1.73 m2. Bodyweight and HbA1c decreased in all subgroups, the latter by − 0.91% to − 0.40% (P < 0.05 vs. baseline). eGFR increased in the 45 to < 60 mL/min/1.73 m2 subgroup (+ 1.42 ± 8.77 mL/min/1.73 m2; P = 0.006). It decreased in the ≥ 90 and 60 to < 90 mL/min/1.73 m2 subgroups (− 8.27 ± 13.73 and − 1.22 ± 10.34 mL/min/1.73 m2; P < 0.001), but not to < 60 mL/min/1.73 m2. In conclusion, there were no new or unexpected safety findings in Japanese patients treated with ipragliflozin for T2DM, and long-term sustained improvements in HbA1c and bodyweight were observed regardless of the presence of renal impairment.


Similar content being viewed by others
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease: a systematic review and meta-analysis. PLoS ONE. 2016;11(7):e158765-e.
Masakane I, Nakai S, Ogata S, Kimata N, Hanafusa N, Hamano T, Wakai K, Wada A, Nitta K. An overview of regular dialysis treatment in Japan (As of 31 December 2013). Ther Apher Dial. 2015;19(6):540–74.
Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, Rodgers A, Mahaffey KW, Cannon CP, Perkovic V, Jardine MJ, Neal B. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e014908.
Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–84.
Heerspink HJL, Karasik A, Thuresson M, Melzer-Cohen C, Chodick G, Khunti K, Wilding JPH, Garcia Rodriguez LA, Cea-Soriano L, Kohsaka S, Nicolucci A, Lucisano G, Lin FJ, Wang CY, Wittbrodt E, Fenici P, Kosiborod M. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8(1):27–35.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Investigators CT. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15(5):463–73.
Zhang X, Zhong Z, Li Y, Li W. Long-term renal outcomes associated with sodium glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2020;2020:e3303.
Takeuchi M, Ogura M, Minoura T, Inagaki N, Kawakami K. Comparative effectiveness of sodium-glucose cotransporter-2 inhibitors versus other classes of glucose-lowering medications on renal outcome in type 2 diabetes. Mayo Clin Proc. 2020;95(2):265–73.
Kitada M, Hirai T, Koya D. Significance of SGLT2 inhibitors: lessons from renal clinical outcomes in patients with type 2 diabetes and basic researches. Diabetol Int. 2020;11:245–51.
Ito Y, Van Schyndle J, Nishimura T, Sugitani T, Kimura T. Characteristics of patients with diabetes initiating sodium glucose co-transporter-2 inhibitors (SGLT2i): real-world results from three administrative databases in Japan. Diabetes Ther. 2019;10(2):549–62.
Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014;74(5):611–7.
Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, Osawa H, Taguchi A, Tanizawa Y, Tobe K, Yoshioka N. Japanese clinical practice guideline for diabetes 2019. Diabetology Int. 2020;11(3):165–223.
Watada H. Current understanding of the effect of sodium-glucose co-transporter-2 inhibitors in Asian patients with diabetes mellitus. Diabetol Int. 2020;11:242–4.
Kadokura T, Akiyama N, Kashiwagi A, Utsuno A, Kazuta K, Yoshida S, Nagase I, Smulders R, Kageyama S. Pharmacokinetic and pharmacodynamic study of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Diabetes Res Clin Pract. 2014;106(1):50–6.
Kashiwagi A, Takahashi H, Ishikawa H, Yoshida S, Kazuta K, Utsuno A, Ueyama E. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17(2):152–60.
Maegawa H, Tobe K, Nakamura I, Uno S. Safety and effectiveness of ipragliflozin in elderly versus non-elderly Japanese type 2 diabetes mellitus patients: 12 month interim results of the STELLA-LONG TERM study. Curr Med Res Opin. 2019;35(11):1901–10.
Maegawa H, Tobe K, Tabuchi H, Nakamura I. Baseline characteristics and interim (3-month) efficacy and safety data from STELLA-LONG TERM, a long-term post-marketing surveillance study of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice. Expert Opin Pharmacother. 2016;17(15):1985–94.
Maegawa H, Tobe K, Tabuchi H, Nakamura I, Uno S. Safety and efficacy of ipragliflozin in elderly versus non-elderly Japanese patients with type 2 diabetes mellitus: a subgroup analysis of the STELLA-LONG TERM study. Expert Opin Pharmacother. 2018;19(4):327–36.
Nakamura I, Maegawa H, Tobe K, Tabuchi H, Uno S. Safety and efficacy of ipragliflozin in Japanese patients with type 2 diabetes in real-world clinical practice: interim results of the STELLA-LONG TERM post-marketing surveillance study. Expert Opin Pharmacother. 2018;19(3):189–201.
Nakamura I, Maegawa H, Tobe K, Uno S. Safety and effectiveness of ipragliflozin for type 2 diabetes in Japan: 12-month interim results of the STELLA-LONG TERM post-marketing surveillance study. Adv Ther. 2019;36(4):923–49.
Nakamura I, Tobe K, Maegawa H, Uno S. Safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus: 24-month interim results of the STELLA-LONG TERM post-marketing surveillance study. Jpn Pharmacol Ther. 2019;47(11):1765–89.
Tabuchi H, Maegawa H, Tobe K, Nakamura I, Uno S. Effect of ipragliflozin on liver function in Japanese type 2 diabetes mellitus patients: a subgroup analysis of the STELLA-LONG TERM study (3-month interim results). Endocr J. 2019;66(1):31–41.
Tobe K, Maegawa H, Nakamura H, Uno S. Safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus stratified by body mass index: a subgroup analysis of 24-month interim reports from the STELLA-LONG TERM post-marketing surveillance study. Jpn Pharmacol Ther. 2019;47(11):1791–805.
Tobe K, Maegawa H, Tabuchi H, Nakamura I, Uno S. Impact of body mass index on the efficacy and safety of ipragliflozin in Japanese patients with type 2 diabetes mellitus: a subgroup analysis of 3-month interim results from the specified drug use results survey of ipragliflozin treatment in type 2 diabetic patients: long-term use study. J Diabetes Investig. 2019;10(5):1262–71.
Grams ME, Sang Y, Ballew SH, Matsushita K, Astor BC, Carrero JJ, Chang AR, Inker LA, Kenealy T, Kovesdy CP, Lee BJ, Levin A, Naimark D, Pena MJ, Schold JD, Shalev V, Wetzels JFM, Woodward M, Gansevoort RT, Levey AS, Coresh J. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. 2019;30(9):1746–55.
Nakamura I, Maegawa H, Tobe K, Uno S. Real-world evidence for long-term safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus: final results of a 3-year post-marketing surveillance study (STELLA-LONG TERM). https://doi.org/10.1080/14656566.2020.1817388(Epub ahead of print).
Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(2):S165–71.
Matsuba I, Kawata T, Iemitsu K, Asakura T, Amemiya H, Ishikawa M, Ito S, Kaneshiro M, Kanamori A, Kubota A, Shinoda K, Takai M, Takuma T, Takihata M, Takeda H, Tanaka K, Matsuzawa Y, Machimura H, Minagawa F, Minami N, Mokubo A, Miyakawa M, Terauchi Y, Tanaka Y. Effects of ipragliflozin on the development and progression of kidney disease in patients with type 2 diabetes: an analysis from a multicenter prospective intervention study. J Diabetes Investig. 2020. https://doi.org/10.1111/jdi.13248.
Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol. 2020;19(1):98.
Hollander P. The role of anti-obesity drugs in patients with type 2 diabetes. US Endocrinology. 2013;9(2):101–7.
Kumagai T, Ota T, Tamura Y, Chang WX, Shibata S, Uchida S. Time to target uric acid to retard CKD progression. Clin Exp Nephrol. 2017;21(2):182–92.
Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165(7):742–8.
Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism. 1997;46(10):1162–5.
Haque T, Rahman S, Islam S, Molla NH, Ali N. Assessment of the relationship between serum uric acid and glucose levels in healthy, prediabetic and diabetic individuals. Diabetol Metab Syndr. 2019;11:49.
Herman JB, Medalie JH, Goldbourt U. Diabetes, prediabetes and uricaemia. Diabetologia. 1976;12(1):47–52.
Chino Y, Samukawa Y, Sakai S, Nakai Y, Yamaguchi J, Nakanishi T, Tamai I. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391–404.
Tanaka M, Yamakage H, Inoue T, Odori S, Kusakabe T, Shimatsu A, Satoh-Asahara N. Beneficial effects of ipragliflozin on the renal function and serum uric acid levels in Japanese patients with type 2 diabetes: a randomized, 12-week, open-label, active-controlled trial. Intern Med. 2020;59(5):601–9.
Acknowledgements
The authors thank all the participants in this study. This study was funded by Astellas Pharma Inc. Medical writing support was provided by Atsuko Yamazaki, PhD, and Tracy Harrison, of inScience Communications, Springer Healthcare, and was funded by Astellas Pharma Inc.
Author information
Authors and Affiliations
Contributions
TK, MH and US contributed to the study design, data analysis and data interpretation. NI contributed to the study design, study conduct, data collection, data analysis and data interpretation. All authors contributed to writing the manuscript and approved the final draft for submission.
Corresponding author
Ethics declarations
Conflict of interest
Tobe K received lecture fees from MSD K.K., Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co. Ltd.; grants from Daiichi Sankyo Co. Ltd., Ono Pharmaceutical Co. Ltd., Takeda Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., MSD K.K., Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Eli Lilly Japan K.K., Asahi Kasei Pharma Corporation, The Mitsubishi Foundation, and Suntory Global Innovation Center Ltd. Maegawa H has received lecture fees from MSD K.K., Sanofi K.K., Astellas Pharma Inc., Nippon Boehringer Ingelheim Co. Ltd., Takeda Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., Astra Zeneca K.K., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd. and Sumitomo Dainippon Pharma Co. Ltd.; research support from Astellas Pharma Inc., Astra Zeneca K.K., Nippon Boehringer Ingelheim Co. Ltd., Sunstar Inc., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co. Ltd., Nissan Chemical Corporation and MIKI Corporation; grants from Takeda Pharmaceutical Co. Ltd., Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Daiichi Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Kowa Pharmaceutical Co. Ltd., Taisho Pharma Co. Ltd., Ono Pharmaceutical Co. Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co. Ltd., Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd., Bayer Yakuhin Ltd., Teijin Pharma Limited, Shionogi & Co. Ltd., Novartis Pharma K.K. and Nipro Corporation. Nakamura I and Uno S are employees of Astellas Pharma Inc.
Ethical standards and human rights statement
This study was conducted as a special drug use surveillance study in compliance with Japanese Good Post-marketing Study Practice (GPSP) and the study protocol was approved by the Japanese government Ministry of Health, Labour and Welfare. All medical institutions that agreed to provide data signed a contract with Astellas Pharma Inc.
Informed consent
Anonymous patient data were collected via electronic survey forms from clinical settings, thus it was not deemed necessary to obtain patient informed consent.
Data sharing statement
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Tobe, K., Maegawa, H., Nakamura, I. et al. Safety and effectiveness of ipragliflozin in Japanese patients with type 2 diabetes mellitus and impaired renal function: subgroup analysis of a 3-year post-marketing surveillance study (STELLA-LONG TERM). Diabetol Int 12, 181–196 (2021). https://doi.org/10.1007/s13340-020-00470-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-020-00470-6