Abstract
Aim
This retrospective study was undertaken to clarify the characteristics of patients in whom vildagliptin exerts its HbA1c-lowering effect fully. The safety of vildagliptin was also examined.
Methods
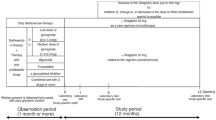
Vildagliptin was administered as initial, additive, or exchange medication to 139 type 2 diabetic patients. Patients were excluded if they had a change in another oral hypoglycemic agent within 24 weeks after starting vildagliptin. The primary efficacy endpoint was the percentage of patients who attained HbA1c level <7 % at week 24.
Results
Vildagliptin significantly increased the percentage of patients who attained HbA1c level <7 %. It was significantly higher in patients with lower baseline HbA1c, higher C-peptide immunoreactivity (CPR) index [plasma CPR (ng/ml)/glucose (mg/dl) × 100], and in patients whose CPR index increased after the administration of vildagliptin. Categorical analyses by age, gender, and body mass index showed no significant differences among categories. There was no significant difference in HbA1c-lowering effect in the categories of oral hypoglycemic agents used concomitantly with vildagliptin, nor of hypoglycemic agents switched to vildagliptin. Logistic regression analyses indicated that the only factor contributing to the glucose-lowering effect of vildagliptin was baseline HbA1c. There were no changes in clinical parameters thought to be adverse events.
Conclusions
Administration of vildagliptin provided a significant and clinically relevant HbA1c-lowering effect irrespective of age, gender, BMI, and concomitantly or previously used hypoglycemic agents. Furthermore, not only high baseline CPR index, but also the increase in CPR index may be associated with the efficacy of this medication.





Similar content being viewed by others
References
Richter B, Banderia-Echtler E, Bergerhoff K, et al. Emerging role of dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–68.
Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91:4612–9.
Mari A, Sallas WM, He YL, et al. Vildagliptin, a dipeptidyl-peptidase IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab. 2005;90:4888–94.
Balas B, Baig MR, Watson C, et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab. 2007;92:1249–55.
He YL, Yamaguchi M, Tarao S, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in Japanese patients with Type 2 diabetes. Int J Clin Pharm Ther. 2010;48:582–95.
Bergman AJ, Stevens C, Zhou YY, et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72.
Ahren B, Schweizer A, Dejager S, et al. Mechanisms of action of the dipeptidyl peptidase-4 inhibitor vildagliptin in humans. Diabetes Obes Metab. 2011;13:775–83.
Marfella R, Barbieri M, Grella R, et al. Effects of vildagliptin twice daily vs. sitagliptin once daily on 24-hour acute glucose fluctuations. J Diabetes Complicat. 2010;24:79–83.
Keating GM. Vildagliptin a review of its use in type 2 diabetes mellitus. Drugs. 2010;70:2089–112.
Signorovitch JE, Wu EQ, Swallow E, et al. Comparative efficacy of vildagliptin and sitagliptin in Japanese patients with type 2 diabetes mellitus. A matching-adjusted indirect comparison of randomized trials. Clin Drug Invest. 2011;31:665–74.
Aroda VR, Henry RR, Han J, et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247–58.
Monami M, Ahren B, Dicembrini I, et al. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15:112–20.
Saisho Y, Miyakoshi K, Tanaka M, et al. Beta cell dysfunction and its clinical significance in gestational diabetes. Endocr J. 2010;57:973–80.
The Japan Diabetes Society. Evidence-based practice guideline for the treatment of diabetes in Japan. Tokyo: Nankodo; 2004 (in Japanese).
Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Horio M, Imai E, Yasuda Y, et al. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56:32–8.
Eposito K, Cozzolino D, Bellastella G, et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2011;13:594–603.
Kikuchi M, Haneda M, Koya D, et al. Efficacy and tolerability of vildagliptin as an add-on to glimepiride in Japanese patients with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2010;89:216–23.
Garber AJ, Foley JE, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with sulfonylurea. Diabetes Obes Metab. 2008;11:1047–56.
Maeda H, Kubota A, Tanaka Y, et al. The safety, efficacy and predictors for HbA1c reduction of sitagliptin in the treatment of Japanese type 2 diabetes. Diabetes Res Clin Pract. 2012;95:20–2.
Bando Y, Kanehara H, Aoki K, et al. Obesity may attenuate the HbA1c-lowering effect of sitagliptin in Japanese type 2 diabetic patients. J Diabetes Invest. 2012;3:170–4.
Prato SD, Barnett AH, Huisman H, et al. Effect of linagliptin monotherapy on glycaemic control and markers of & #x03B2;-cell function in patients with inadequately controlled type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2011;13:258–67.
Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26:881–5.
Mari A, Scherbaum WA, Nilsson PM, et al. Characterization of the influence of vildagliptin on model-assessed & #x03B2;-cell function in patients with type 2 diabetes and mild hyperglycemia. J Clin Endocrinol Metab. 2008;93:103–9.
Goto A, Takaichi M, Kishimoto M, et al. Body mass index, fasting plasma glucose levels, and C-peptide levels as predictors of the future insulin use in Japanese type 2 diabetic patients. Endocr J. 2010;57:237–44.
Asano T, Kawamura M, Watanabe T, et al. Indices of urinary and serum C-peptide corrected with fasting plasma glucose for decision-making of insulin therapy in type 2 diabetes-validation and comparison. J Jpn Diabetes Soc. 2008;51:759–63 (in Japanese).
Saisho Y, Kou K, Tanaka K, et al. Postprandial serum C-peptide to plasma glucose ratio as a predictor of subsequent insulin treatment in patients with type 2 diabetes. Endocr J. 2011;58:315–22.
Mannucci E, Rotella CM. Future perspectives on glucagon-like peptide-1 and cardiovascular risk. Nutr Metab Cardiovasc Dis. 2008;18:639–45.
Giorgino F, Leonardini A, Natalicchio A, et al. Multifactorial intervention in Type 2 diabetes: the promise of incretin-based therapies. J Endocrinol Invest. 2011;34:69–77.
Monami M, Lamanna C, Desideri CM, et al. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29:14–25.
Acknowledgments
H.I. has received lecture fees from MSD and Novartis, and research grants from Sanofi-Aventis, Pfizer, Dainippon Sumitomo, Teijin, Takeda, Tanabe Mitsubishi, MSD, Daiichi Sankyo, Roche, and Eli Lilly. The other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tanaka, M., Sekioka, R., Nishimura, T. et al. Serum C-peptide to plasma glucose ratio may be associated with efficacy of vildagliptin in Japanese patients with type 2 diabetes mellitus. Diabetol Int 6, 197–205 (2015). https://doi.org/10.1007/s13340-014-0186-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-014-0186-7