Abstract
Infectious bronchitis (IB) is a highly infectious avian pathogen, which affects the respiratory tract, gut, reproductive system, and kidney of chicks of all ages. Many different serotypes of IB virus (IBV) are recognized which cause different clinical manifestations. According to the antigenic differences, different serotypes of the virus do not cross-protect. Massachusetts serotype induces the best cross-protection against other serotypes. Recently, the IBV QX strain has been detected in Iran. QX strain causes permanent damage to the oviduct if it occurs in the early life cycle and is a significant factor in layer and breeder chicken flocks. In this study, we compare the H120 and Ma5 vaccines’ protection against early challenge with the QX strain in commercial chicks. one-day-old commercial chicks were divided into six groups. Groups 1 and 2 were unvaccinated groups. Groups 3 and 5 were vaccinated with the H120 vaccine (eye drop) and groups 4 and 6 were vaccinated with Ma5 (eye drop) on the 6th day (5 days after vaccination). Groups 2, 3 and 4 challenged (oculonasal) with QX strain (10^4 EID50). Ciliostasis test, histopathology, and quantitative real-time RT-PCR were done at 11 days-old of age. Results showed that neither H120 nor Ma5 could induce proper cross-protection against QX early challenge, but the viral load and adverse pathological records in vaccinated chicks were less than that in the non-vaccinated groups. It can be concluded that vaccination on the first day of the life of a chick offers not full protection against the IBV QX strain but reduced the viral load and pathological damages in vaccinated chickens. Applying other forms of vaccination and using different genotypes on one-day-old chicks are suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Infectious bronchitis virus (IBV) represents one of the most relevant infectious diseases of poultry, causing severe economic losses mainly associated with respiratory and reproductive syndromes, decreased productive performances and increased mortality. Even if biosecurity and good management practices are fundamental in the disease containment, widespread vaccination is also essential to control the disease [8]. IBV belongs to the order of Nidovirales, family Coronaviridae and to the genus of Gamma-coronavirus. Different serotypes have been reported worldwide and new variant serotypes continue to be recognized [12]. In September 1997, an outbreak of the disease (QX outbreak), characterized mainly by swelling of the stomach (proventriculitis), diarrhea and loss of body weight in 25–70-day-old chickens occurred in chicken flocks in Qingdao, China [19]. Genotyping of IBV strains isolated in Iran were classified into seven distinct phylogenetic groups (Mass, 793/B like IS/1494 like, IS/720-like, QX-like, IR-1, and IR-2) based on analysis of mainly HVRs of the S1 gene [9, 13]. Bozorgmehri-Fard et al. (2014) have demonstrated the presences of QX viruses in Iranian commercial flocks and after this outbreak, we have some many false layers in Iran. If the QX enter to flock before 10 days old, it causes a permanent effect on oviduct and blind layers. As QX vaccine doesn’t have a permit to use in Iranian flock, just Massachusetts and 793/B like vaccines are using. Nevertheless, no cross-protection studies have been done if QX enters to flock before 10 days, what is happening? The aim of the study is comparison of two types of Massachusetts—like commercial avian infectious bronchitis (IB) vaccines in an early challenge against IBV QX strain in commercial chicken.
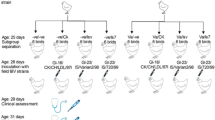
90 day-old commercial broiler chicks with IBV maternally derived antibodies (MDA) were obtained from a commercial hatchery. Chicks were kept in an isolation unit with filtered air under negative pressure. 1-day-old commercial chicks were divided into six groups. Chicks were divided into six groups of 15 birds (Table 1). Groups 1 and 2 were unvaccinated groups. Groups 3 and 5 were vaccinated with the H120 vaccine (eye drop) and groups 4 and 6 were vaccinated with Ma5 (eye drop) on the 6th day (5 days after vaccination). On the 6th day (5 days after vaccination), groups 2, 3 and 4 challenged (ocular-nasal) with QX like genotype (10^4 EID50). In 11 days-old of age ciliostasis test, histopathology and quantitative real-time RT-PCR were done. QX- like IBV isolate (at fifth egg passages) was selected from the virus bank, faculty of veterinary medicine, University of Tehran. The S1 sequence of challenge virus was submitted to the NCBI (accession number: KT583570.1). The antibody level of experimental chicks tested before the challenge was measured using ELISA (IDEXX). Five days after the challenge, level of protection of the trachea was examined using a ciliostasis test on 10 tracheal ring explants per chicken. Immediately after the removal, the trachea was stored in DMEM. The level of ciliostasis was determined by low-power microscopy and scored as follows: 0 = all cilia beating, 1 = 75% beating, 2 = 50% beating, 3 = 25% beating, and 4 = none beating (100% ciliostasis). The protection against the IBV challenge was determined and calculated [15]. Viral RNA was isolated from trachea samples using Cinna Pure RNA extraction kit (Sinaclone, Iran) recommended by the supplier. cDNA synthesized using RevertAid first strand cDNA synthesis Kit (Thermo Scientific, Canada). Real-time PCR assay in order to amplify a conserved sequence within the 5′-UTR of the IBV genome and 28 s ribosomal-RNA as a reference gene was used. A downstream primer IBV5′GU391 (5′-GCTTTTGAGCCTAGCGTT-3′, nt 391-408), an upstream primer IBV5′GL533 (5′GCCATGTTGTCACTGTCTATTG-3′, nt 512-533), and a Taqman® dual-labelled probe IBV5′G (5′-FAMCACCACCAGAACCTGTCACCTC-BHQ1-3′, nt 473-494) were used to amplify a 143-bp fragment of 5′-UTR [3]. To amplify a 61-bp fragment of 28 s rRNA gene, we used forward primer (5′-GGCGAAGCCAGAGGAAACT-3′), reverse primer (5′-GACGACCGATTTGCACGTC-3′) and probe (FAM- AGGACCGCTACGGACCTCCACCA-TAMRA) [16]. The 20 μl real-time PCR reaction contained 2 μl AMS 10X PCR Buffer (Sinaclon, Iran), 1 μl dNTP mix (Sinaclon, Iran), 0.8 μl Mgcl2 (50 mM) (Sinaclon, Iran), 0.2 μl CinnaGen Taq DNA polymerase (Sinaclon, Iran), 5 μl template cDNA, primers to a final concentration of 0.1 μM and probe to a final concentration of 0.1 μM and nuclease-free water. The reaction was carried out in a QIAGEN Rotor-Gene Q (Corbett Rotor-Gene 6000) (USA, CA). PCR cycling parameters were 95 °C for 2 min, then 40 cycles of 95 °C for 15 s and 60 °C for 1 min. As the specific primers for QX detection were not available and differentiation between challenge and mass type virus was not possible, so we normalized the challenged viral loads using groups 5 and 6 (Vaccinated and unchallenged groups) viral load. Tissue samples from trachea from chicks were removed and fixed in buffered formalin (10%), processed, sectioned, and stained with hematoxylin and eosin (H & E) for histopathological evaluation. To evaluate and compare the tracheal pathological scores and ciliostasis between the groups Mann–Whitney test using SPSS 12.1 software was used. The significance level was considered 0.02. Finally, one-way ANOVA with a significance level of 0.05 was used to compare viral load.
According to serological results from ELISA kit, the antibody titer of chickens is around 42,150 with cv 32% so it shows antibody titers uniformity. There is a meaningful difference in tracheal viral load between vaccinated and control groups (ANOVA test) (Table 1). In spite of lower viral load in a vaccinated group with H120 than the other with Ma5, the difference is not significant (Table 1). According to pathologic tracheal lesion score, lesions of vaccinated groups are lower than the control group. There is a significant difference in H120 vaccinated group because of its efficiency. There is not a significant difference between these two groups for tracheal ciliostasis score (Table 1). For this test, protection rate of H120 vaccinated group is 74% and in the other is 71%, so there is no difference between them (Table 1).
If QX IBV infected the chickens during the first 10 days, has the permanent effect on oviduct and causing the false layers. All cross-protection studies have done according to the evaluation of chickens after 14–21 days after vaccination when the humeral antibody level is insufficient level. Commercial 793/B serotype vaccines are available from several companies including 4/91 (Intervet), IB88 (Merial), Ibird (CEVA Sante animale). In addition, some QX type IBV vaccines are have been produced by some companies (L1148 (Zeotis) & D388 (Intervet)). Nevertheless, the main question is current vaccines can prevent QX IBV circulation strain or need QX specific vaccine? Sarueng et al. did a study on the efficacy of live IBV programs against infection by QX like IBV. The experimental groups were divided into 5 groups. Group 1 broilers were administered with Poulvac IB QX-like at 1 day old. Group 2 broilers were administered with Poulvac IB QX-like at 1 and 14 days old. Group 3 broilers were administered with Nobilis IB H120 and Poulvac IB QX-like at 1 and 14 days old, respectively. Group 4 broilers were administered with Nobilis IB H120 and Nobilis IB 4–91 at 1 and 14 days old, respectively. Group 5 served as a positive control group. All groups were challenged with IBV THA80151. Although different vaccination programs were used in this study, all the vaccinated groups demonstrated protection against damage after the challenge with live IB QX-like virus. The vaccines were able to decrease the tracheal rales, ciliary activity and histological lesion scores in the vaccinated groups compared with the positive control group but the vaccination programs could not prevent infection because IB QX-like virus was still re-isolated from some infected organs. The booster vaccine with the heterologous strain was able to increase the cross-protection of IBV QX-like challenge [17]. In a study by De Wit et al., on commercial broiler breeders, it was reported that Mass (Day 0) + 793/B (Day 14) vaccines combination produced %51 protection in cilliostasis test 5 days post challenge with D388 (QX strain) at 28th day, while the protection was increased to %74 at 7th days post challenge [7]. Hawk and Jones studied the membranous immune response induced by IBV after rinsing the trachea completely. It was indicated that IgA level of the tracheal fluid would be high for 6 h, then decreased gradually in 9 h and finally decreased significantly. Fredrick studied the level of protection against the IBV by vaccinating on days 1, 7 and 14 days old and found out that serum and lacrimal secretion IgA and IgG levels on chicks vaccinated on one-day old was lower than levels induced by vaccinating on day 7 and especially 14-days old chicks due to immaturity of the immune system [18]. Hence, the inflammation and scavenging level of the epithelial cells and goblet cells was higher in one-day vaccinated chicks than those vaccinated on 7 and 4 days old after challenging with IB field virus. According to this, vaccinating 7 days post-hatch is recommended due to immaturity of the immune system and the availability of IgY maternal antibody [18]. Also, it was showed in De Wit’s study that the level of IgM antibody against IB was lower one-day-old chicks were vaccinated than those are older [6]. Faez (2015) studied the immune responses and reactions of SPF chicks after administering 3 live vaccines of ND, IB and aMPV. It was shown that all HI antibody titers of all groups were protective and the aMPV vaccine suppressed aMPV ELISA titers when administered with other vaccines [1]. Cellular and membranous immunity induced by single or combined administration of ND, IB and aMPV vaccines significantly increased in CD4+, CD8+ and B cells containing IgA of the tracheal samples of vaccinated groups compared with non-vaccinated ones. The difference was not significant among the vaccinated groups. Simultaneous vaccination of ND, IB and aMPS live vaccines was not effected on induced protection against aMPV and IBV [1]. In Chhabra and Ganapathy study on the host response to predict IBV’s intensity and tropism, it was indicated that IS-885 nephropathogenic and QX strains, first identified in Israel, induce more apoptosis in renal cell culture media compared with M41 strain [4]. However, the M41 strain could induce more apoptosis in tracheal tissue culture media than nephropathogenic IS-885 and QX strain. It was also showed that enhancing the response of the innate immune system has resulted in the tissue tropism of various IBV strains [4]. It was indicated in Okino’s study on cellular and humoral immune responses against IBV after vaccinating with various doses of live attenuated vaccines on one-day-old chicks. The results showed IgG and IgA antibody levels in lacrimal secretions and trachea were significantly increased 5 days after vaccination (together with expressing cellular immunity genes such as interferon, CD8+lymphocyte T marker, and homologue granzyme A). However, when the vaccine was not administered in full dose, incomplete immunity response was recorded. It was indicated in this study that IgA, IgG and CD8+T cell responses induced after full-dose IB vaccination in membranes were well correlated with antiviral protective immunity. This study emphasized on the full dose of the vaccine since, like previous studies, appropriate protective immunity was completely depended on the vaccine dose [14]. In Faez and Ganapathy study, the ciliostasis protection percentage against Middle East 885 and 1494 middle east IBV strains were 80 and 60, respectively when a group of broiler chicks received H120 vaccine on 1 day old and then CR88 vaccine on 14 days old. However, when H120 and CR88 vaccines were administered on one-day old and then CR88 vaccine were administered on 14-day old, the protection rate against tracheal ciliostasis by Middle East 885 and 1494 IBV strains was more than 80% while 100% protection was noticed in case of clinical signs and tracheal and renal lesions even with 19–21% genetic difference between S1 gene of vaccine standard strains and challenged wild variants [2]. It was indicated in Kahya’s study that administering H120 vaccine one time on one-day-old chicks could not immunize them against Middle East 885 and 1494 IBV strains resulting in finding renal and respiratory lesions [11]. Jackwood also indicated that making the vaccine of these two IBV variants is time-consuming and costly [10]. Cool et al. indicated that vaccination with only one serotype could not induce full immunity against heterologous IBV strains but higher immunity could be achieved using a combination of various strains of IBV live vaccines, proctotypes, against the acute heterologous IBV variants [5]. No study has been conducted to study the local immunity in early challenges. In routine cross protection methods, the chicks are challenged 3 weeks after the last IB vaccine when expressing the antibody but this could not be an appropriate assay for the field conditions. The results of the study indicated that if the field IBV infected the chicks in the first week of the life and the chicks were vaccinated on one day old using H120 or Massachusetts IB vaccines, the chicks could not achieve to full protection level but the viral load and pathological damages could be decreased resulting in relative protection against IBV. It is suggested to follow the same study plan with 793/B or 793/B+ Mass vaccines and vaccinate one-day-old chicks to assess the protection against QX strain and other common IBV genotypes.
Change history
19 February 2018
In the original publication, there is an error in the article title. It should be corrected as ‘Efficacy of H120 and Ma5 avian infectious bronchitis vaccines in early challenge against QX strain’. Also, ‘OX strain’ should be changed to ‘QX strain’ throughout the article.
References
Awad F, Forrester A, Baylis M, Lemiere S, Jones R, Ganapathy K. Immune responses and interactions following simultaneous application of live Newcastle disease, infectious bronchitis and avian metapneumovirus vaccines in specific-pathogen-free chicks. Res Vet Sci. 2015;98:127–33.
Awad F, Forrester A, Baylis M, Lemiere S, Ganapathy K. Protection conferred by live infectious bronchitis vaccine viruses against variant Middle East IS/885/00-like and IS/1494/06-like isolates in commercial broiler chicks. Vet Rec Open. 2015;2(2):e000111.
Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, et al. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138(1–2):60–5. https://doi.org/10.1016/j.jviromet.2006.07.018.
Chhabra R, Kuchipudi SV, Chantrey J, Ganapathy K. Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses. Virology. 2016;488:232–41.
Cook JK, Orbell SJ, Woods MA, Huggins MB. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28(5):477–85.
De Wit J, Swart W, Fabri T. Efficacy of infectious bronchitis virus vaccinations in the field: association between the α-IBV IgM response, protection and vaccine application parameters. Avian Pathol. 2010;39(2):123–31.
De Wit J, Cook JK, Van Der Heijden HM. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40(3):223–35.
Franzo G, Tucciarone CM, Blanco A, Nofrarías M, Biarnés M, Cortey M, et al. Effect of different vaccination strategies on IBV QX population dynamics and clinical outbreaks. Vaccine. 2016;34(46):5670–6.
Hosseini H, Bozorgmehri Fard MH, Charkhkar S, Morshed R. Epidemiology of avian infectious bronchitis virus genotypes in Iran (2010–2014). Avian Dis. 2015;59(3):431–5.
Jackwood MW, Hilt DA, Brown TP. Attenuation, safety, and efficacy of an infectious bronchitis virus GA98 serotype vaccine. Avian Dis. 2003;47(3):627–32.
Kahya S, Coven F, Temelli S, Eyigor A, Carli KT. Presence of IS/1494/06 genotype-related infectious bronchitis virus in breeder and broiler flocks in Turkey. Ankara universitesi veteriner fakültesi dergisi. 2013;60(1):27–31.
Najafi H, Langeroudi A, Hashemzadeh M, Karimi V, Madadgar O, Ghafouri S, et al. Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014–2015. Arch Virol. 2015;16:1–10. https://doi.org/10.1007/s00705-015-2636-3.
Najafi H, Langeroudi AG, Hashemzadeh M, Karimi V, Madadgar O, Ghafouri SA, et al. Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014–2015. Arch Virol. 2016;161:1–10.
Okino CH, Alessi AC, Montassier MDFS, Rosa AJDM, Wang X, Montassier HJ. Humoral and cell-mediated immune responses to different doses of attenuated vaccine against avian infectious bronchitis virus. Viral Immunol. 2013;26(4):259–67.
Pohuang T, Sasipreeyajan J. Efficacy of live H120 strain against variant genotype infectious bronchitis virus Isolated in Thailand in broiler chickens. Thai J Vet Med. 2015;45(3):411.
Rothwell L, Young JR, Zoorob R, Whittaker CA, Hesketh P, Archer A, et al. Cloning and characterization of chicken IL-10 and its role in the immune response to Eimeria maxima. J Immunol. 2004;173(4):2675–82.
Sarueng E, Wanasawaeng W, Sasipreeyajan J, Chansiripornchai N. Efficacy of Live infectious bronchitis vaccine programs against infection by QX-like strain of infectious bronchitis virus. Thai J Vet Med. 2014;44(2):187–94.
van Ginkel FW, Padgett J, Martinez-Romero G, Miller MS, Joiner KS, Gulley SL. Age-dependent immune responses and immune protection after avian coronavirus vaccination. Vaccine. 2015;33(23):2655–61.
Yudong W, YongLin W, Zichun Z, GenChe F, Yihai J, Xiange L, et al. Isolation and identification of glandular stomach type IBV (QX IBV) in chickens. Chin J Anim Quar. 1998;15(1):1–3.
Acknowledgements
This study was conducted under a grant of research council, University of Tehran (No. 7508013/6/12). The authors gratefully acknowledge Mr Behrooz Asadi for his extensive technical supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: “The article title ′OX strain′ has been changed to ′QX strain′ and also ′OX strain′ has been changed to ′QX strain′ throughout the article”.
A correction to this article is available online at https://doi.org/10.1007/s13337-018-0420-1.
Rights and permissions
About this article
Cite this article
Karimi, V., Ghalyanchilangeroudi, A., Hashemzadeh, M. et al. Efficacy of H120 and Ma5 avian infectious bronchitis vaccines in early challenge against QX strain. VirusDis. 29, 123–126 (2018). https://doi.org/10.1007/s13337-017-0414-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-017-0414-4