Abstract
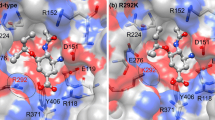
Human infection with H7 influenza subtypes usually resulted in mild disease with a rare mortalities, however, human infection with the avian low pathogenic H7N9 influenza virus resulted in about 38.6 % human fatality. Due to the new cross-species barrier of this virus subtype, there is an urgent need to better understand the susceptibility to commercially available antivirals and their relation to the structural changes of the viral neuraminidase. Neuraminidases derived from 2013 H7N9, H5N1 and H1N1 were subjected to a structural analysis of their catalytic and framework binding sites. The modeling structure of selected neuraminidases from H7N9 and influenza A subtypes were solved and the docking studies with oseltamivir, zanamivir, laninamivir and peramivir were conducted. The active site residues that are responsible for both binding and cleavage of the terminally linked sialic acid receptors were found conserved. Docking studies with oseltamivir, zanamivir, laninamivir and peramivir revealed that the laninamivir and peramivir showed superior energy binding activities in comparison to the commonly used oseltamivir and zanamivir. The results presented in the current study provide data that are useful for the future treatment of different influenza A subtypes including the recently emerged H7N9.


Similar content being viewed by others
References
Cardona CJ, Xing Z, Sandrock CE, Davis CE. Avian influenza in birds and mammals. Comp Immunol Microbiol Infect Dis. 2009;32(4):255–73.
Chong AK, Pegg MS, Taylor NR, von Itzstein M. Evidence for a sialosyl cation transition-state complex in the reaction of sialidase from influenza virus. Eur J Biochem. 1992;207(1):335–43.
Colman PM. New antivirals and drug resistance. Annu Rev Biochem. 2009;78:95–118.
Du QS, Wang SQ, Chou KC. Study of drug resistance of chicken influenza A virus (H5N1) from homology-modeled 3D structures of neuraminidases. Biochem Biophys Res Commun. 2007;354:634–40.
Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;. doi:10.1056/NEJMoa1304459.
Gubareva LV. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 2004;103:199–203.
Gubareva LV, Webster RG, Hayden FG. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother. 2001;45:3403–8.
Horimoto T, Kawaoka Y. Pandemic threat posed by avian influenza A viruses. Clin Microbiol Rev. 2001;14(1):129–49.
Hurt AC, Selleck P, Komadina N, Shaw R, Brown L, Barr IG. Susceptibility of highly pathogenic A (H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Res. 2007;73:228–31.
Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–65.
Kohno SH, Kida H, Mizuguchi M, Shimada J. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010;54(11):4568–74.
Koopmans M, Wilbrink B, Conyn M, Natrop G, van der Nat H, Vennema H, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363(9409):587–93.
Kumar S, Tamura K, Jakobsen IB, Nei M. Molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–5.
Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, Nguyen KH, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108.
Le MT, Wertheim HF, Nguyen HD, Taylor W, Hoang PV, Vuong CD, et al. Influenza A H5N1 clade 2.3.4 virus with a different antiviral susceptibility profile replaced clade 1 virus in humans in northern Vietnam. PLoS ONE. 2008;3(10):e3339.
McKimm-Breschkin JL. Resistance of influenza viruses to neuraminidase inhibitors-a review. Antiviral Res. 2000;47:1–17.
Murphy BR, Webster RG. Orthomyxoviruses. Fields Virology. Philadelphia: Lippincott-Raven; 1996.
Russell J, Haire F, Stevens J, Collins J, Lin P, Blackburn M, et al. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature. 2006;443:45–9.
Shobugawa Y, Saito R, Sato I, Kawashima T, Dapat C, Dapat IC, et al. Clinical effectiveness of neuraminidase inhibitors–oseltamivir, zanamivir, laninamivir, and peramivir–for treatment of influenza A(H3N2) and A(H1N1)pdm09 infection: an observational study in the 2010-2011 influenza season in Japan. J Infect Chemother. 2012;18(6):858–64.
Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci USA. 2012;109(11):4269–74.
Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9(10):e1003657.
von Itzstein M. The war against influenza: discovery and development of sialidase inhibitors. Nat Rev Drug Discov. 2007;6:967–74.
Wang K, Shun-Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children (published trials only). Cochrane Database Syst Rev. 2012;18(4):CD002744.
Watanabe A, Chang SC, Kim MJ, Chu DWS, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis. 2010;51(10):1167–75.
Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubo S. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother. 2009;53(1):186–92.
Yen HL, Hoffmann E, Taylor G, Scholtissek C, Monto AS, Webster RG, et al. Importance of neuraminidase active-site residues to the neuraminidase inhibitor resistance of influenza viruses. J Virol. 2006;80(17):8787–95.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eweas, A.F., Abdel-Moneim, A.S. In-silico structural analysis of the influenza A subtype H7N9 neuraminidase and molecular docking with different neuraminidase inhibitors. VirusDis. 26, 27–32 (2015). https://doi.org/10.1007/s13337-014-0245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-014-0245-5