Abstract
Background and Objectives
Palovarotene is under development for the treatment of fibrodysplasia ossificans progressiva (FOP). The objectives of this study were to evaluate palovarotene pharmacokinetics under fed versus fasted conditions and its induction potential towards cytochrome P450 3A4 (CYP3A4) substrate, midazolam.
Methods
In this phase I, open-label trial (NCT04829773), palovarotene pharmacokinetics were characterized after repeated once-daily dosing. In one cohort, healthy participants received three doses of palovarotene 20 mg on Days 1, 6, and 11, as whole capsules under fasted or fed conditions, or sprinkled on food under fed conditions. In another cohort, individuals received midazolam 2 mg on Days 1 and 15 and a daily dose of palovarotene 20 mg on Days 2–15. Palovarotene and midazolam pharmacokinetics, including area under the concentration-time curve from time zero to infinity (AUC(0–∞)) and maximum observed plasma drug concentration (Cmax), were assessed. Adverse events (AEs) were recorded.
Results
Overall, 23 participants completed each part. Palovarotene Cmax and AUC(0–∞) increased by 16.5% and 39.7% under fed versus fasted conditions. Pharmacokinetics were comparable between the whole capsule and sprinkled on food, under fed conditions. Midazolam AUC(0–∞) and Cmax decreased by 13.3% and 9.7% upon palovarotene co-administration over 14 days, less than that required to be considered a weak CYP3A4 inducer. Plasma palovarotene exposures were comparable after single and multiple doses. No serious AEs were reported.
Conclusions
These data support palovarotene administration after a meal, as a whole capsule or sprinkled on food. Palovarotene at 20 mg/day is a not a clinical inducer of CYP3A4. These results provide insights into palovarotene pharmacokinetics, aiding optimization of administration for patients with FOP.
Clinical Trials Registration Number
NCT04829773.
Plain Language Summary
Fibrodysplasia ossificans progressiva, also known as FOP, is a very rare genetic condition where bone forms in places it is not usually found, such as in the muscles, tendons, and ligaments. Retinoids are molecules that the body produces from vitamin A to aid normal bone development. Palovarotene is a therapeutic retinoid that has been developed for the treatment of FOP. This article describes a clinical trial where people without FOP received oral palovarotene to determine how it is absorbed and broken down (metabolized) by the body when taken after a meal or after fasting (a period of not eating) as a whole capsule or when sprinkled on food. The trial also examined how palovarotene might interact with other treatments that are broken down by the body in the same way as palovarotene.
The trial found that the amount of palovarotene that circulates in the blood increased more when taken after a meal compared with after fasting. Palovarotene was metabolized by the body in a similar way when taken as a whole capsule or when sprinkled on food. This finding is important as some people with FOP have difficulty swallowing. At a 20 mg dose, palovarotene was unlikely to interact with other treatments that are metabolized in the same way. No serious side effects were reported.
These results show that palovarotene should be taken after a meal, either as a whole capsule or sprinkled on food.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Palovarotene, a cytochrome P450 3A4 substrate, is under development for the treatment of fibrodysplasia ossificans progressiva, an ultra-rare genetic condition |
This trial demonstrated that palovarotene 20 mg/day is not a clinical inducer of cytochrome P450 3A4 |
These data support palovarotene administration after a meal, as a whole capsule or sprinkled on food |
1 Introduction
Fibrodysplasia ossificans progressiva (FOP) is an ultra-rare genetic disorder caused by a gain-of-function pathogenic variant of the activin receptor-like kinase-2/activin A receptor type-1 (ALK2/ACVR1) gene, which encodes a bone morphogenetic protein (BMP) type I receptor. This variant leads to aberrant activation of the BMP signaling pathway [1, 2], resulting in progressive heterotopic ossification within soft and connective tissues [3, 4]. Heterotopic ossification is often preceded by painful, recurrent flare-ups and results in progressive restriction of movement, cumulative disability, and decreased quality of life [5, 6]. Until recently, there were no disease-modifying treatments for FOP [7]. Therapeutic approaches were limited to supportive care and symptomatic management of flare-ups [8,9,10]. Palovarotene is an orally bioavailable, selective retinoic acid receptor gamma agonist [11], which is thought to act by inhibiting the BMP signaling pathway, and ultimately heterotopic ossification [12, 13]. Palovarotene has been found to prevent new heterotopic ossification in patients with FOP in clinical trials, with results from phase II (NCT02190747) and phase III (NCT03312634) trials indicating efficacy [14, 15]. Palovarotene has been approved for use in adults and children (females 8 years and older, males 10 years and older) with FOP [7, 16].
The pharmacokinetics of palovarotene have been studied in previous trials in healthy participants and patients with emphysema [11, 17,18,19]. At doses of 0.2, 0.5, and 1 mg, the mean steady-state maximum plasma drug concentration (Cmax) and area under the concentration-time curve (AUC) values of palovarotene for 14–16 days were dose-proportional [19]. However, the highest anticipated clinical dose for patients with FOP is 20 mg, as a flare-up treatment regimen [7]. Therefore, Part 1 of this trial was conducted to evaluate the pharmacokinetics and relative bioavailability of a single daily oral dose of palovarotene 20 mg, under both fed and fasted conditions, in healthy participants. Furthermore, as patients with FOP may experience ankylosis of the jaw [20], severely limiting its movement and resulting in swallowing difficulties, palovarotene bioavailability was evaluated when swallowed as a whole capsule versus sprinkled on soft food under fed conditions.
In humans, based on the low projected efficacious plasma concentrations (138–202 ng/ml) at the maximal dose of 20 mg and low unbound plasma levels (97.5–99% bound), palovarotene is not likely to inhibit the cytochrome P450 3A4 (CYP3A4)-dependent elimination of other drugs [7]. Patients with FOP may receive concomitant medications that are CYP3A4 substrates, and studies performed in vitro have indicated that palovarotene may induce CYP3A4. Part 2 of this trial investigated the induction potential of palovarotene 20 mg towards CYP3A4 using midazolam as a probe substrate. The pharmacokinetics of palovarotene with repeated administration at the higher clinical dose of 20 mg were also characterized. Both parts of the trial evaluated the safety and tolerability of palovarotene 20 mg.
2 Methods
This trial was approved by the IntegReview Institutional Review Board and carried out in compliance with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines [21, 22].
2.1 Trial Participants
This phase I, open-label trial (NCT04829773) was conducted at a single center in the US from 3 January 2019 to 29 March 2019. The trial was composed of two parts, with each part consisting of separate cohorts of 24 healthy adult participants. Participants were eligible if they were aged 18–55 years, had a body mass index (BMI) of 18–30 kg/m2, body weight of ≥ 50 kg, resting pulse of 45–100 beats per minute (bpm), and systolic/diastolic blood pressures of < 140/90 mmHg. Exclusion criteria included: history or current evidence of a clinically significant or uncontrolled disease, exposure to retinoids in the 30 days preceding provision of informed consent, and exposure to an investigational drug within 30 days or 6 half-lives (whichever was greater) prior to the first dose of palovarotene. The full inclusion and exclusion criteria are outlined in Supplementary Table 1.
Baseline and safety measurements were conducted at screening and monitored throughout the trial. Assessments included medical history, physical examination, vital signs, 12-lead electrocardiograms (ECGs), and clinical laboratory parameters. Females of child-bearing potential were required to have a negative blood serum pregnancy test prior to receiving palovarotene. Pregnancy testing was also conducted on Days−1, 5, and 10, with positive results leading to discontinuation of palovarotene. Prior medications were recorded at screening and concomitant medications were assessed throughout the trial. Baseline and safety measurements are described in further detail in Supplementary Information 1. All participants provided written and signed informed consent before engaging in any trial procedure.
2.2 Trial Design
2.2.1 Part 1
In Part 1, all participants received three single oral doses of palovarotene 20 mg on Days 1, 6, and 11, separated by a 5-day washout. Each dose was administered as two 10-mg powder-filled hard gelatin capsules, swallowed whole or sprinkled on applesauce. Final assessments, including blood samples for pharmacokinetic analysis of palovarotene, were taken on Day 13. Crossover dosing was used in this trial; participants were randomized 1:1:1 to one of three different treatment sequences: A-B-C, B-C-A, or C-A-B (Fig. 1a). Treatment A consisted of palovarotene whole capsules administered after a 10-h overnight fast, swallowed with 237 ml of water; a standardized meal, containing 800–1200 kilocalories, was provided 4 h after dosing. Treatment B consisted of palovarotene whole capsules administered 30 min after the start of a standardized high-fat, high-calorie breakfast, swallowed with 237 ml water. Treatment C consisted of palovarotene sprinkled on 1 teaspoon of applesauce, administered 30 min after the start of a standardized high-fat, high-calorie breakfast. The standardized breakfast for treatments B and C contained approximately 800–1000 calories, consisting of approximately 150, 250, and 500–600 calories from protein, carbohydrate, and fat, respectively.
Study design: A Crossover dosing for each treatment sequence in Part 1 and B dosing regimen of palovarotene and midazolam in Part 2. PK pharmacokinetic. aOne participant in the BCA treatment sequence completed Day 1 dosing of palovarotene 20 mg (Treatment B) and was discontinued on Day 5 because of a positive amphetamine drug screen. bFollowing administration of midazolam. cFollowing administration of palovarotene
2.2.2 Part 2
In Part 2, participants received a single dose of midazolam on the morning of Day 1. On Day 2 (after a 24-h midazolam blood draw) through Day 15, participants received a single dose of palovarotene 20 mg every morning. On Day 15, a second dose of midazolam was administered immediately following the palovarotene dose (Fig. 1b). Each 20 mg dose of palovarotene was administered as two 10-mg powder-filled capsules swallowed whole. Each dose of midazolam was 2 mg, administered as a syrup. All doses were taken 30 min after the start of a standardized high-fat, high-calorie breakfast. The breakfast contained approximately 800–1200 kilocalories.
2.3 Preparation of Samples
As palovarotene is sensitive to light, blood samples were collected and processed under conditions that minimized light exposure. Plasma was separated and frozen until shipping for analysis. For each sample, 2 ml blood was drawn.
2.3.1 Part 1
Blood samples for pharmacokinetic analysis of palovarotene in plasma were obtained on: Days 1, 6, and 11 prior to dose (Hour 0); Hours 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 16 post-palovarotene administration; Days 2, 7, and 12 at 24 h post-palovarotene administration; Days 3, 8, and 13 at 48 h post-palovarotene administration.
2.3.2 Part 2
Blood samples for pharmacokinetic analysis of midazolam and its metabolite 1-hydroxymidazolam were obtained on Days 1 and 15 at the following time points: pre-dose (0 h) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after midazolam or palovarotene administration, respectively, and on Days 2 and 16 at 24 h after midazolam administration. Blood samples were taken following palovarotene administration on Day 15 since both palovarotene and midazolam were administered. Blood samples for pharmacokinetic analysis of palovarotene were obtained on Days 2 and 15 at the following time points after palovarotene administration: pre-dose (0 h) and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, and 16 h, on Days 3 and 16 at 24 h after palovarotene administration, and on Day 17 at 48 h after palovarotene administration. For assessment of steady state, blood samples for pharmacokinetic analysis of palovarotene were obtained pre-dose on Days 13, 14, and 15.
2.4 Trial Endpoints
2.4.1 Part 1
The primary objective was to assess the pharmacokinetics and relative bioavailability of palovarotene under fasted conditions versus after a high-fat, high-calorie meal and when administered under fed conditions by two oral methods: whole capsule or sprinkled on soft food. The pharmacokinetic parameters assessed were Cmax, time to reach Cmax (tmax), and AUC from time zero to the last quantifiable time point (AUC(0–last)) and from time zero to infinity (AUC(0–∞)). The terminal rate constant (λz), apparent terminal elimination half-life (t1/2), apparent volume of distribution after oral administration (Vd/F), and apparent total clearance of the drug from plasma after oral administration (CL/F) were also assessed.
2.4.2 Part 2
The pharmacokinetic parameters assessed for midazolam were Cmax, tmax, AUC from time zero to 24 h (AUC(0–24h)), AUC(0–last), and AUC(0–∞). Additional pharmacokinetic parameters included λz, t1/2, and CL/F. The pharmacokinetic parameters for 1-hydroxymidazolam were calculated as Cmax, tmax, AUC(0–24h), and AUC(0–last). The pharmacokinetic parameters for palovarotene on Day 2, after the first dose, were calculated as Cmax, tmax, and AUC(0–24h). The last concentration before the next study drug administration at steady state (Ctrough,ss) was observed on Days 13 through 15. The pharmacokinetic parameters examined for palovarotene on Day 15 at steady state included Cmax,ss, minimum plasma concentrations (Cmin,ss), tmax,ss, Vd,ss/F, and CLss/F. Additional pharmacokinetic parameters included AUC from time zero to 24 h, calculated by linear-log trapezoidal summation (AUCτ), λz, t1/2, and the accumulation ratio (Rac).
2.5 Safety Assessments
The safety and tolerability of palovarotene in healthy participants were evaluated in both parts of the study. Safety evaluation included a descriptive analysis of adverse events (AEs), serious AEs (SAEs), and treatment-emergent AEs (TEAEs; AEs occurring following the first dose of palovarotene), coded according to the Medical Dictionary for Regulatory Activities (MedDRA, version 21.0) and reported by preferred term (PT) throughout the trial for all participants. If any mucocutaneous effects were observed, symptomatic therapy was permitted, including analgesics, emollients, lip moisturizers, artificial tears, and topical steroids. The investigator may have also recommended prophylactic use of these therapies at the start of palovarotene treatment. If the AE remained intolerable, palovarotene was to be discontinued. TEAEs were rated according to severity assessments of mild (events that are easily tolerated with no disruption of normal daily activity), moderate (events that cause sufficient discomfort to interfere with daily activity and/or require a simple dose medication), and severe (events that incapacitate and prevent usual activity or require systemic drug therapy or other treatment). Safety endpoints also included ECG, vital signs (temperature, respiratory rate, blood pressure, and heart rate), physical examination, body weight and height, BMI, and laboratory parameters (hematology, clinical chemistry, and urinalysis). Any changes compared with baseline assessed as clinically significant were recorded as an AE.
2.6 Statistical Analyses
All statistical analyses were performed using the software SAS® (version 9.4, Cary, NC). Both absolute values and change from baseline values for each participant were given where applicable. Baseline was the last non-missing available measurement prior to the first dose of palovarotene. Data were summarized by each treatment group. Continuous variables were summarized using the number of non-missing observations (n), arithmetic mean (mean), standard deviation (SD), coefficient of variation (CV), geometric mean (GeoMean), median, minimum, and maximum; categorical variables were summarized using the frequency count and the percentage of participants in each category. Pharmacokinetic variables were calculated from the plasma concentrations using Phoenix WinNonlin™ software (version 8.0 or later; Certara USA, Inc. Princeton, NJ).
2.6.1 Part 1
Statistical comparisons were performed using the pharmacokinetic evaluable population, which includes those who completed the trial and did not have any major protocol deviations that may have confounded interpretation of the results. An analysis of variance (ANOVA) was used to assess AUC(0–∞) and Cmax, whereas AUC(0–last), tmax, CL/F, λz, and t½ were summarized descriptively. All statistical tests were performed two-sided and were carried out at an alpha level of 0.05 unless otherwise stated. No adjustment for multiple comparisons was employed.
The relative bioavailability of oral palovarotene under fed versus fasted conditions administered as a whole capsule was assessed using 90% confidence intervals (CIs) obtained within the framework of the ANOVA for the ln-transformed parameters Cmax and AUC(0–∞). The effect of food was assessed from the 90% CI for the ratio of population GeoMeans between fed and fasted treatments (based on ln-transformed data) using the conventional equivalence limits of 80% and 125% for Cmax and AUC(0–∞). The relative bioavailability of oral palovarotene under fed conditions sprinkled on applesauce versus swallowed as a whole capsule was also assessed using 90% CIs obtained within the framework of the ANOVA for the ln-transformed parameters Cmax and AUC(0–∞). Administration under fed conditions, sprinkled (Treatment C: Test) or as a whole capsule (Treatment B: Reference), was considered bioequivalent if the 90% CI for the ratio of population GeoMeans between sprinkled and swallowed treatment as reference (based on ln-transformed data) was contained in the conventional equivalence limits of 80% and 125% for Cmax and AUC(0–∞).
2.6.2 Part 2
Statistical analyses were conducted to assess changes in midazolam Cmax and AUC(0–∞) following administration of 14 daily doses of palovarotene at Day 15 compared with the Cmax and AUC(0–∞) before palovarotene administration at Day 1. An ANOVA was performed on ln-transformed Cmax and AUC(0–∞), whereas AUC(0–last), tmax, CL/F, λz, and t½ were summarized, descriptively. If 90% CIs for ln-transformed Cmax and AUC(0–∞) were within 80% and 125%, the absence of a significant drug-drug interaction (DDI) was concluded.
For palovarotene, individual plasma concentrations over time and pharmacokinetic parameters from Days 2 and 15 were summarized for the Pharmacokinetic evaluable population using descriptive statistics (see Supplementary Information 2 for further details on population analysis sets). Mean palovarotene concentration-time profiles were presented (linear scale). Steady-state analysis was performed on the log-transformed pre-dose Ctrough concentrations (pre-dose concentrations on Days 13 through 15) using Helmert contrasts. Further statistical information for both parts is summarized in Supplementary Information 2.
3 Results
3.1 Trial Participants
Of the participants enrolled, 23/24 in both parts (95.8%) completed the trial. Demographic and baseline characteristics are outlined in Table 1. During the trial, 12 participants in Part 1 received concomitant medications, summarized in Supplementary Table 2. In Part 2, one participant received prior medications before and during the trial, and 21 participants received concomitant medications during the trial, of which the most common were emollients and protectives (83.3%), antihistamines including fexofenadine (62.5%), and topical corticosteroids, such as fluocinolone acetonide (45.8%). None of the prior or concomitant medications, in either part, were expected to impact the trial outcomes.
3.2 Pharmacokinetic Evaluations
3.2.1 Part 1
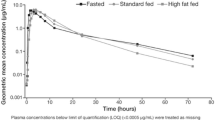
Following administration under fed conditions (after a high-fat, high-calorie breakfast), plasma palovarotene concentration-time profiles showed an increase in concentration compared with fasted conditions (Fig. 2). Additionally, under fed conditions, when the contents of the palovarotene capsule were sprinkled on applesauce, the plasma concentration-time profiles were comparable with swallowing the capsule whole. Individual plasma pharmacokinetic parameters for palovarotene demonstrated that the GeoMean Cmax was higher under fed conditions following administration of the palovarotene capsules whole (142.5 ng/ml) or sprinkled on applesauce (133.4 ng/ml) compared with fasted conditions (whole capsule, 122.3 ng/ml) (Table 2). The GeoMean AUC(0–∞) was also comparable under fed conditions between administration of the capsules whole and sprinkled on applesauce (1055.9 h·ng/ml versus 1063.0 h·ng/ml), whereas it was lower under fasted conditions (whole capsule, 760.4 h·ng/ml). The GeoMean CL/F was lower under fed conditions for both capsules whole (18.9 l/h) and sprinkled (18.8 l/h) compared with fasted conditions (26.3 l/h), which reflects the higher AUC(0–∞) under fed conditions. Statistical analyses of palovarotene bioavailability showed that following administration of palovarotene capsules swallowed whole under fed conditions, there was a 39.7% increase in AUC(0–∞) and a 16.5% increase in Cmax compared with administration under fasted conditions (Table 3). Under fed conditions, AUC(0–∞) and Cmax were comparable following palovarotene administration as whole capsules versus sprinkled and were considered bioequivalent.
Mean (SD) palovarotene plasma concentrations (pg/ml) over time in Part 1. Pharmacokinetic population (N = 24): All participants who received at least one dose of study drug and had evaluable plasma concentration-time profiles. A: Fasted, capsule: two palovarotene 10-mg capsules after at least a 10-h overnight fast; B: Fed, capsule: two palovarotene 10-mg capsules 30 min after the start of a high-fat, high-calorie breakfast; C: Fed, sprinkled: two palovarotene 10-mg capsules opened and sprinkled on 1 teaspoon of applesauce 30 min after the start of a high-fat, high-calorie breakfast. Three single oral doses of palovarotene 20 mg on Days 1, 6, and 11 were separated by 5-day washout periods
3.2.2 Part 2
Mean concentrations for midazolam (Fig. 3a) and 1-hydroxymidazolam (Fig. 3b) were slightly lower after 14 daily doses of palovarotene 20 mg compared with midazolam administered alone. Plasma pharmacokinetic parameters demonstrated that the GeoMean Cmax for midazolam and 1-hydroxymidazolam was slightly lower in the presence of palovarotene (5.04 ng/ml and 2.08 ng/ml) compared with midazolam administered alone (5.58 ng/ml and 2.27 ng/ml) (Table 4). Additionally, in the presence of palovarotene, the GeoMean AUC(0–∞) for midazolam was lower than for midazolam administration alone (28.1 h·ng/ml vs 32.4 h·ng/ml). The GeoMean ratio (%) for AUC(0–∞) and Cmax was 86.7% (90% CI: 79.8, 94.1) and 90.3% (90% CI: 83.7, 97.5), representing a 13.3% and 9.7% reduction in midazolam exposure between co-administration of midazolam (Day 15) and palovarotene and midazolam monotherapy (Day 1) (Table 5).
Mean plasma concentrations over time in Part 2: A midazolam (ng/ml), B 1-hydroxymidazolam (ng/ml), and C palovarotene (pg/ml). M midazolam (Day 1), P palovarotene (Days 2–14), Q combination of midazolam and palovarotene (Day 15). Pharmacokinetic population: All participants who received at least one dose of study drug and had evaluable plasma concentration-time profiles
Mean plasma palovarotene concentrations were comparable between Day 2 after a single dose of palovarotene and Day 15 after multiple doses of palovarotene (Fig. 3c). The GeoMean Cmax on Day 2 (139.0 ng/ml) was comparable with the Cmax,ss on Day 15 (132.8 ng/ml) (Table 6). The GeoMean AUC(0–24h) on Day 2 (856.8 h·ng/ml) was comparable to the AUCτ on Day 15 (892.1 h·ng/mL), and the Rac was estimated to be 1.04. Pre-dose GeoMean Ctrough concentrations (90% CI) were 3.5 (2.9, 4.2) ng/ml, 3.4 (2.8, 4.1) ng/ml, and 3.6 (3.0, 4.4) ng/ml on study Days 13, 14, and 15, respectively; differences were not significant between Days 13 and 14 (p = 0.76), or Days 14 and 15 (p = 0.25).
3.3 Safety Assessments
Participants received all intended doses for the trial across both parts with the exception of two individuals. One individual discontinued from Part 1 because of a positive amphetamine drug screen, and another individual who discontinued from Part 2 because of an AE relating to a skin burning sensation, which was considered related to the study treatment.
In Part 1, overall, 20/24 participants (83.3%) had ≥ 1 TEAE (Table 7). No deaths or SAEs, AEs leading to discontinuation, or other significant AEs were reported. Six (26.1%) and 12 (50.0%) participants who were administered whole capsules experienced ≥ 1 TEAE under fasted and fed conditions. Eleven participants (47.8%) experienced ≥ 1 TEAE when the contents of the capsule were sprinkled on applesauce under fed conditions. Overall, 39 TEAEs were recorded for the 20 participants, 35 of which were considered by the investigator to be related to the study treatment. Mild TEAEs were reported by 17 participants (70.8%), while one moderate TEAE was reported (4.2%). No severe TEAEs were reported. TEAEs reported by PT included dry skin (50.0%), headache (20.8%), pruritus (12.5%), dandruff (8.3%), skin exfoliation (8.3%), dizziness (8.3%), and dry lips (8.3%) (Table 7). Although there were some recorded ECG abnormalities, none were determined by the investigator to be AEs or clinically significant, and there were no apparent consistent safety findings related to vital signs. No hematology, clinical chemistry, liver enzyme, lipase or amylase, lipid profile, or urinalysis abnormalities were assessed as clinically significant.
In Part 2, overall, 22/24 participants (91.7%) reported ≥ 1 TEAE; 90 mild or moderate TEAEs were reported and 86 of these were considered by the investigator to be related to the study treatment (Table 7). No deaths or SAEs were reported. No TEAEs were reported on Day 1 after only receiving midazolam and prior to palovarotene dosing on Day 2. Mild TEAEs were reported by 22 participants (91.7%), and moderate TEAEs were reported by 9 participants (37.5%); no severe TEAEs were described. Moderate TEAEs occurred only between Days 2 and 14. TEAEs reported by PT included dry skin (83.3%), pruritus (50.0%), dry mouth (33.3%), and generalized pruritus (25.0%) (Table 7).
Other AEs included headache (8.3%), hyperesthesia (4.2%), and blurred vision (8.3%); these were mild in severity with the exception of one headache, which was considered moderate. Due to the low incidence and severity of these AEs, they were not considered clinically significant.
No abnormalities in vital signs, ECGs, or laboratory parameters were assessed as clinically significant. TEAEs recorded in physical examinations included dry lips, face, scalp, and skin, flaky skin, peeling skin, small skin lesion, and skin desquamation and exfoliation, all of which were considered clinically significant.
4 Discussion
This phase I, open-label trial in healthy individuals investigated the effect of food and administration method on the pharmacokinetics and bioavailability palovarotene and the induction potential of palovarotene on the CYP3A4 substrate midazolam in vivo. Pharmacokinetic data presented here provide insights that have informed optimal palovarotene administration options as a treatment for patients with FOP.
The pharmacokinetics of a single oral dose of palovarotene 20 mg under fed and fasted conditions, and when administered as whole capsules versus sprinkled on soft food under fed conditions, were first characterized. Plasma palovarotene AUC(0–∞) and Cmax were 39.7% and 16.5% higher, respectively, following a high-fat, high-calorie meal compared with administration under fasted conditions. This finding confirms the positive influence of food on the bioavailability of the palovarotene capsule formulation, supporting administration after a meal, and is consistent with results for other retinoid medications for which the presence of food in the gastrointestinal tract increases bioavailability [23,24,25]. In terms of the mechanism for this higher bioavailability when administered with food, similar to other retinoids, palovarotene is highly lipophilic (logP 6.8) [26]. Bioavailability of lipophilic compounds is generally increased by high-fat food, and therefore increased bioavailability of palovarotene is expected here given the participants had consumed high-fat, high-calorie meals.
Furthermore, plasma palovarotene AUC(0–∞) and Cmax were comparable when palovarotene was administered as a whole capsule and when sprinkled onto soft food, demonstrating bioequivalence between these administration methods. For patients receiving palovarotene who cannot swallow capsules whole, such as individuals with jaw ankylosis caused by FOP, sprinkling of capsule contents could offer an alternative. These findings provide valuable insight into different administration methods for palovarotene that could be utilized in subsequent clinical studies and/or clinical practice for future management of FOP and have informed the FDA label and Health Canada Product Monograph of palovarotene [7, 16].
This trial also evaluated the CYP3A4 induction potential of palovarotene using midazolam as a probe substrate. According to Food and Drug Administration (FDA) guidance on clinical DDI studies, medications are classified as weak CYP inducers if they decrease the AUC of the probe substrate by ≥ 20 to < 50% [27]. The reduction in AUC(0–∞) for midazolam upon co-administration of palovarotene at a dose of 20 mg for 14 days was 13%. Therefore, at this dose, palovarotene is not considered a clinical inducer or inhibitor of CYP3A4 and is unlikely to interact with therapeutics that are metabolized by CYP3A4. Notably, in this study, the focus was on enzyme induction by palovarotene as a perpetrator drug. However, DDIs due to enzyme inhibition affecting PK of palovarotene as a victim drug should also be considered. For example, it is known that ketoconazole, a strong inhibitor of CYP3A4, increases the Cmax and AUC of palovarotene by approximately 121% and 212%, respectively [7]. It is therefore advised to avoid concomitant use of strong CYP3A4 inhibitors such as azole antifungals (e.g., ketoconazole, itraconazole) and macrolide antibiotics (e.g., clarithromycin) with palovarotene treatment [7]. Similarly, coadministration of palovarotene with strong CYP3A4 inducers (e.g., carbamazepine, phenytoin, rifabutin, St John’s wort extract) should also be avoided [7].
Participants during both parts of the trial received concomitant medications, and none of these medications were expected to impact trial outcomes. Fexofenadine, an antihistamine received by many participants in the trial, is a substrate of P-glycoprotein (P-gp) and organic-anion-transporting polypeptides (OATPs) [28]. CYP3A4 and P-gp often share the same substrate, and concomitant use of fexofenadine with P-gp inhibitors or inducers can affect fexofenadine exposure. However, according to in vitro data, palovarotene is not considered a substrate or inhibitor of P-gp [29]. Regarding other transporter proteins, in vitro data indicate that palovarotene is not a substrate of breast cancer resistance protein (BCRP), OATP1B1 and OATP1B3, or organic cation transporter 1 (OCT1) [29]. Regarding inhibition of these transporters (BCRP, OATP1B1, OATP1B3, OCT1, and bile salt export pump [BSEP]), clinically relevant interaction of palovarotene as a perpetrator drug with other drugs is unlikely considering the low exposure of palovarotene at the maximal clinical dose of 20 mg [29].
The pharmacokinetic profile of palovarotene following single and multiple doses of the highest anticipated clinical dose of 20 mg was also characterized. The non-significant differences in pre-dose Ctrough concentrations confirmed that the pharmacokinetic analyses on Day 15 were conducted at steady state. The plasma concentrations between Days 2 and 15 of treatment were comparable, and the Rac of 1.04 suggested little or no accumulation of palovarotene upon repeated once-daily dosing, informing appropriate dosing regimens for patients with FOP.
Safety assessments demonstrated that a single dose of palovarotene 20 mg was generally well tolerated in healthy individuals when administered with or without food and by whole capsule or sprinkled on food. When palovarotene was administered under fasted conditions, a lower proportion of participants reported TEAEs compared with fed conditions, an expected finding as fasted participants had lower systemic exposure to palovarotene than fed participants. The majority of TEAEs were mucocutaneous effects (including dry skin and pruritus) of mild severity and resolved with symptomatic treatments, consistent with findings from phase II and III studies [30, 31] and literature recommending the use of prophylactic therapies to manage retinoid-associated mucocutaneous TEAEs [32]. Palovarotene at a dose of 20 mg was also well tolerated when co-administered with midazolam; mild TEAEs were observed, most frequently during palovarotene dosing between Days 2 and 14.
The standardized diet used during Part 1 of this trial was selected since meals that are high in total calories and fat content are likely to elicit a potential effect on the bioavailability of a drug. However, given that high-calorie and high-fat meals may not represent a real-world diet, the generalizability of these results to the wider population of patients with FOP should be considered. Generalizability may also be limited since this trial was conducted at a single center in the US. However, pharmacokinetic phase I trials are typically conducted at a single site, and an ethnic sensitivity trial found no differences in the pharmacokinetics and safety of palovarotene between healthy Japanese and non-Japanese participants [33].
5 Conclusions
The results of this trial confirm the positive influence of food on the bioavailability of palovarotene and support administration after a meal. Furthermore, administration of palovarotene sprinkled on food is considered bioequivalent to swallowing palovarotene capsules whole. Additionally, palovarotene 20 mg is unlikely to be a clinical inducer or inhibitor of CYP3A4 and can therefore be administered alongside concomitant medications that are classified as CYP3A4 substrates. There was also little or no accumulation of palovarotene upon repeated once-daily dosing. These findings further elucidated the pharmacokinetic profile of palovarotene and informed the administration method and dosing regimens in phase II trials and the pivotal phase III trial of palovarotene in patients with FOP; these trials formed the basis of the approval of palovarotene for the treatment of FOP [7, 16].
References
Hüning I, Gillessen-Kaesbach G. Fibrodysplasia ossificans progressiva: clinical course, genetic mutations and genotype-phenotype correlation. Mol Syndromol. 2014;5(5):201–11. https://doi.org/10.1159/000365770.
Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6(9):518–27. https://doi.org/10.1038/nrrheum.2010.122.
Kaplan FS, Tabas JA, Gannon FH, et al. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Jt Surg Am. 1993;75(2):220–30. https://doi.org/10.2106/00004623-199302000-00009.
Pignolo RJ, Bedford-Gay C, Liljesthröm M, et al. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J Bone Miner Res. 2016;31(3):650–6. https://doi.org/10.1002/jbmr.2728.
Connor JM, Evans DA. Fibrodysplasia ossificans progressiva. The clinical features and natural history of 34 patients. J Bone Jt Surg Br. 1982;64(1):76–83. https://doi.org/10.1302/0301-620x.64b1.7068725.
Nakahara Y, Kitoh H, Nakashima Y, et al. Longitudinal study of the activities of daily living and quality of life in japanese patients with fibrodysplasia ossificans progressiva. Disabil Rehabil. 2019;41(6):699–704. https://doi.org/10.1080/09638288.2017.1405083.
Sohonos Product Monograph. 2022. https://pdf.hres.ca/dpd_pm/00064435.PDF. Accessed 13 June 2022.
Di Rocco M, Baujat G, Bertamino M, et al. International Physician Survey on Management of FOP: A Modified Delphi Study. Orphanet J Rare Dis. 2017;12(1):110. https://doi.org/10.1186/s13023-017-0659-4.
Kaplan FS, Pignolo RJ, Al Mukaddam M, et al. Genetic disorders of heterotopic ossification. In: Primer on the metabolic bone diseases and disorders of mineral metabolism. Wiley; 2018; p. 865–70.
Kaplan F, Al Mukaddam M, Baujat G, et al. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Proc Intl Clin Council FOP, 1, 2019:24–45. https://d3n8a8pro7vhmx.cloudfront.net/ifopa/pages/1042/attachments/original/1628863428/Authors_and_Consultants_%281%29.pdf?1628863428. Accessed 23 Aug 2023.
Hind M, Stinchcombe S. Palovarotene, a novel retinoic acid receptor gamma agonist for the treatment of emphysema. Curr Opin Investig Drugs. 2009;10(11):1243–50.
De Luca F, Uyeda JA, Mericq V, et al. Retinoic acid is a potent regulator of growth plate chondrogenesis. Endocrinology. 2000;141(1):346–53. https://doi.org/10.1210/endo.141.1.7283.
Shimono K, Tung WE, Macolino C, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat Med. 2011;17(4):454–60. https://doi.org/10.1038/nm.2334.
ClinicalTrials.gov. Identifier: NCT03312634 An Efficacy and Safety Study of Palovarotene for the Treatment of FOP (MOVE), 2017.
Pignolo RJ, Baujat G, Hsiao EC, et al. Palovarotene for fibrodysplasia ossificans progressiva (FOP): results of a randomized, placebo-controlled, double-blind phase 2 trial. J Bone Miner Res. 2022. https://doi.org/10.1002/jbmr.4655.
FDA. 2023. Sohonos highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/215559s000lbl.pdf. Accessed 29 Aug 2023.
Brennan B, Chiu Y, Berthelon L, et al. Effect of age and gender on the pharmacokinetics of R667, a novel agent for the treatment of emphysema, healthy volunteers. J Pharm Pharm Sci. 2007;10(1):9–16.
Brennan BJ, Brown AB, Kolis SJ, et al. Effect of R667, a novel emphysema agent, on the pharmacokinetics of midazolam in healthy men. J Clin Pharmacol. 2006;46(2):222–8. https://doi.org/10.1177/0091270005283836.
Chiu YY, Roth MD, Kolis S, et al. Pharmacokinetics of a novel agent, R667, in patients with emphysema. Br J Clin Pharmacol. 2007;63(5):527–33. https://doi.org/10.1111/j.1365-2125.2006.02808.x.
Herford AS, Boyne PJ. Ankylosis of the jaw in a patient with fibrodysplasia ossificans progressiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96(6):680–4. https://doi.org/10.1016/j.tripleo.2003.08.002.
World Declaration of Helsinki. Ethical principles for medical research involving human subjects. 2013;310(20):2191–4.
ICH. ICH Harmonised Guideline Integrated Addendum to ICH E6(R1): guideline for Good Clinical Practice ICH E6(R2) ICH Consensus Guideline. 2018. https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf. Accessed 23 Aug 2023.
Charman WN, Porter CJ, Mithani S, et al. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the role of lipids and pH. J Pharm Sci. 1997;86(3):269–82. https://doi.org/10.1021/js960085v.
McNamara PJ, Jewell RC, Jensen BK, et al. Food increases the bioavailability of acitretin. J Clin Pharmacol. 1988;28(11):1051–5. https://doi.org/10.1002/j.1552-4604.1988.tb03129.x.
Schmitt-Hoffmann A, Roos B, Sauer J, et al. Influence of food on the pharmacokinetics of oral alitretinoin (9-cis retinoic acid). Clin Exp Dermatol. 2011;36:18–23. https://doi.org/10.1111/j.1365-2230.2011.04033.x.
National Center for Biotechnology Information. PubChem compound summary for CID 10295295, Palovarotene. https://pubchem.ncbi.nlm.nih.gov/compound/Palovarotene. Accessed 25 Aug 2023.
FDA. Clinical Drug interaction studies—cytochrome P450 enzyme- and transporter-mediated drug interactions guidance for industry. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed 23 Aug 2023.
EMA. Fexofenadine hydrochloride 180mg film-coated tablets summary of product characteristics. https://www.medicines.org.uk/emc/product/3488/smpc/print. Accessed 25 Aug 2023.
EMA. Assessment report: Sohons. https://www.ema.europa.eu/en/documents/assessment-report/sohonos-epar-refusal-public-assessment-report_en.pdf. Accessed 25 Aug 2023.
Kaplan FS, Hsiao EC, Baujat G, et al. Palovarotene inhibits the development of new heterotopic ossification in fibrodysplasia ossificans progressiva (FOP). Bone Abstracts. 2019. https://doi.org/10.1530/boneabs.7.OC27.
Pignolo RJ, Al Mukaddam M, Baujat G, et al. Palovarotene (PVO) for fibrodysplasia ossificans progressiva (FOP): data from the phase III MOVE trial. J Bone Miner Res. 2020;35:16–7.
Van de Kerkhof PCM, Layton A. Handbook of systemic drug treatment in dermatology. 2nd ed. Taylor & Francis Group; 2015.
Dube L, Haga N, Grogan D, et al. A pharmacokinetic, safety, and tolerability trial of palovarotene in healthy Japanese and non-Japanese participants. Eur J Drug Metab Pharmacokinet. 2023. https://doi.org/10.1007/s13318-023-00815-x.
Acknowledgements
The authors thank all participants involved in the trial, investigators, and research staff in the participating institution. The authors extend their thanks to Donna Grogan for her contributions to the study, data collection and analysis, and drafting of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This trial was sponsored by Ipsen.
Conflicts of Interest
RM: Employee of Ipsen; LD: Pharmacokinetic Consultant for Ipsen; JO: Employee of Ipsen; K-HLQS: Coordinator of Ipsen FOP-program and MO-trial.
Ethics Approval
The original protocol (version 2.0, dated 14 December 2018; approved 20 December 2018), protocol amendment 1 (dated 7 January 2019; approved 17 January 2019), informed consent forms and updates for this trial were approved by the Institutional Review Board in accordance with pertinent country-specific regulatory requirements, and carried out in compliance with the Declaration of Helsinki and International Council for Harmonisation Good Clinical Practice Guidelines.
Informed Consent
All participants provided written and signed informed consent before engaging in any trial procedure.
Consent for Publication
Not applicable.
Data Availability
Qualified researchers may request access to patient-level study data that underlie the results reported in this publication. Additional relevant study documents, including the clinical study report, study protocol with any amendments, annotated case report form, statistical analysis plan and dataset specifications may also be made available. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of study participants. Where applicable, data from eligible studies are available 6 months after the studied medicine and indication have been approved in the US and EU or after the primary manuscript describing the results has been accepted for publication, whichever is later. Further details on Ipsen's sharing criteria, eligible studies and process for sharing are available here (https://vivli.org/members/ourmembers/). Any requests should be submitted to www.vivli.org for assessment by an independent scientific review board.
Code Availability
Not applicable.
Medical Writing Support
The authors thank Emma Lockyer, MChem, Jessica A. Buttress, PhD, and Oliver Palmer, BSc (Hons), of Costello Medical, UK, for providing medical writing and editorial support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
Author Contributions
Substantial contributions to study conception and design: RM, LD, JO, K-HLQS; substantial contributions to analysis and interpretation of the data: RM, LD, JO, K-HLQS; drafting the article or revising it critically for important intellectual content: RM, LD, JO, K-HLQS; final approval of the version of the article to be published: RM, LD, JO, K-HLQS.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Marino, R., Dube, L., Ogier, J. et al. The Pharmacokinetic Profile of Palovarotene: An Open-Label Phase I Trial Investigating the Effect of Food and Potential for Drug–Drug Interaction in Healthy Participants. Eur J Drug Metab Pharmacokinet 48, 691–707 (2023). https://doi.org/10.1007/s13318-023-00856-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00856-2