Abstract
Background and Objective
Extracorporeal membrane oxygenation (ECMO) is used in critically ill patients that require respiratory and/or cardiac support. Cefiderocol is a novel siderophore antibiotic that may require use in infected critically ill patients supported by ECMO. The objective of this study was to determine the loss of cefiderocol through an ex vivo adult ECMO circuit using a Quadrox-iD oxygenator.
Methods
A 3/8-inch, simulated, ex vivo closed-loop ECMO circuit was prepared with a Quadrox-iD adult oxygenator and primed with fresh whole blood. Cefiderocol was administered into the circuit to achieve a starting concentration of approximately 90 mg/L. Post-oxygenator blood samples were collected at 0, 0.25, 0.5, 1, 2, 4, 6, 12, and 24 h after the addition of the drug to determine the loss in the circuit. A glass control jar was prepared with the same blood matrix and maintained at the same temperature to determine drug degradation. The experiment was conducted in triplicate. The rate of cefiderocol loss in the ECMO circuit was compared with that in the control by one-way analysis of variance.
Results
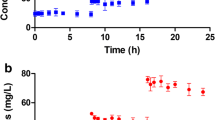
At 0 h, the difference between the pre- and post-oxygenator concentrations was − 4 ± 4% (range 0 to − 7%). After 24 h, the cefiderocol percent reduction was similar between the ECMO circuit and control (50% ± 13 vs. 50% ± 9, p = 1.0).
Conclusions
The degradation rate of cefiderocol did not differ significantly within the ECMO circuit and control, suggesting no loss due to sequestration or adsorption. Pharmacokinetic studies in patients supported by ECMO are warranted to determine final dosing recommendations.

Similar content being viewed by others
References
Bartlett RH, Gattinoni L. Current status of extracorporeal life support (ECMO) for cardiopulmonary failure. Minerva Anestesiol. 2010;76:534–40.
Sherwin J, Heath T, Watt K. Pharmacokinetics and dosing of anti-infective drugs in patients on extracorporeal membrane oxygenation: a review of the current literature. Clin Ther. 2016;38:1976–94.
Shekar K, Roberts JA, McDonald CI, Fisquet S, Barnett AG, Mullany DV, et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16:R194.
Wu JY, Srinivas P, Pogue JM. Cefiderocol: a novel agent for the management of multidrug-resistant gram-negative organisms. Infect Dis Ther. 2020;9:17–40.
Cies JJ, Nikolos P, Moore WS 2nd, Giliam N, Low T, Marino D, et al. Oxygenator impact on meropenem/vaborbactam in extracorporeal membrane oxygenation circuits. Perfusion. 2022;37:729–37.
Shionogi Inc. Cefiderocol (Fetroja) package insert. Florham Park: Shionogi Inc.; 2020.
Shekar K, Roberts JA, McDonald CI, Ghassabian S, Anstey C, Wallis SC, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19:164.
Shekar K, Roberts JA, Barnett AG, Diab S, Wallis SC, Fung YL, et al. Can physiochemical properties of antimicrobials be used to predict their pharmacokinetics during extracoproreal membrane oxygenation? Illustrative data from ovine models. Crit Care. 2015;19:437.
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–82.
Tron C, Leven C, Fillatre P, Maillard N, Nesseler N, Tattevin P, et al. Should we fear tubing adsorption of antibacterial drugs in extracoproreal membrane oxygenation? An answer for cepahlosporins and carbapenems. Clin Exp Pharmacol Physiol. 2016;43:281–3.
Leven C, Fillatre P, Petitcollin A, Verdier MC, Laurent J, Nesseler N, et al. Ex vivo model to decipher the impact of extracorporeal membrane oxygenation on beta-lactam degradation kinetics. Ther Drug Monit. 2017;39:180–4.
Riera J, Domenech L, Garcia S, Pau A, Sosa M, Domench J, et al. Pharmacokinetics of cefiderocol during extracorporeal membrane oxygenation: a case report. Perfusion. 2023. https://doi.org/10.1177/02676591231160462.
Mane C, Delmas C, Porerie J, Jourdan G, Verwaerde P, Marcheix B, et al. Influence of extracorporeal membrane oxygenation on the pharamcokinetics of ceftaroline/tazobactam: an ex vivo and in vivo study. J Transl Med. 2020;19:213.
Lee JH, Lee DH, Kim JS, Jung WB, Heo W, Kim YK, et al. Pharmacokinetics and Monte Carlo simulation of meropenem in critically ill adult patients receiving extracorporeal membrane oxygenation. Front Pharmacol. 2021;12: 768912.
Gijsen M, Dreesen E, Annaert P, Nicolai J, Debaveye Y, Wauters J, et al. Meropenem pharmacokinetics and target attainment in critically ill patients are not affected by extracorporeal membrane exygenation: a matched cohort analysis. Microogranisms. 2021;9:1310.
Acknowledgements
We acknowledge Tyler Ackley, Jennifer Tabor-Rennie, Alissa Padgett, and Elizabeth Cyr for assistance with the conduct of these ex vivo experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was reviewed and approved by the Hartford Healthcare Institutional Review Board. Healthy volunteer participants provided written informed consent to donate blood for the study.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Conflict of Interests
JLK and DPN are members of the Shionogi Speakers’ Bureau and have participated in advisory boards. The remaining authors have nothing to disclose.
Funding
This study was supported by an investigator-initiated trial grant from Shionogi Inc. (Florham Park, NJ). Shionigi Inc. had no role in the study design, conduct, interpretation of data, or writing of the manuscript.
Authors’ Contributions
Study concept and methodology design: JLK, DPN, AC, JAG; protocol writing: JLK; study conduct: AVB, AC, JLK; data analyses: AVB, JLK; data interpretation: AVB, JAG, DPN, JLK; manuscript writing: AVB, JLK; manuscript review: AVB, AC, JAG, DPN, JLK.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Berry, A.V., Conelius, A., Gluck, J.A. et al. Cefiderocol is Not Sequestered in an Ex Vivo Extracorporeal Membrane Oxygenation (ECMO) Circuit. Eur J Drug Metab Pharmacokinet 48, 437–441 (2023). https://doi.org/10.1007/s13318-023-00840-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-023-00840-w