Abstract
Background and Objectives
The treatment of Parkinson’s disease (PD) is still symptomatic since disease-modifying treatments for PD are not available. Oral levodopa is the gold standard for the treatment of PD motor symptoms. However, incomplete and fluctuating plasma exposure of levodopa leads to suboptimal treatment of the symptoms. The main objective of this study was to investigate to what extent increased carbidopa doses (50 and 100 mg) increase the plasma levels of 100-mg immediate-release (IR) levodopa compared to a 25-mg carbidopa dose with and without co-administration of 200 mg entacapone.
Methods
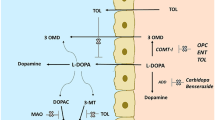
A double-blind, placebo-controlled, randomized, crossover, phase I, pharmacokinetic study with 25 healthy volunteers was conducted. In addition, a semi-mechanistic pharmacokinetic model was built to theoretically evaluate the effect of inhibiting aromatic amino acid decarboxylase (AADC) and catechol-O-methyltransferase (COMT) mediated metabolism of levodopa on the exposure of levodopa.
Results
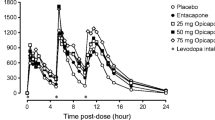
The effect of increased carbidopa doses 50 and 100 mg on the total exposure (AUC) of 100 mg IR levodopa was +29% and +36%, respectively, when entacapone was co-administered. Without entacapone, the corresponding increases were +13% and +17%. With entacapone co-administration, the increased carbidopa dose also clearly increased levodopa trough concentration. There was no significant effect on the peak concentrations of levodopa.
Conclusions
Increasing carbidopa doses significantly increased the exposure and reduced the fluctuation of IR levodopa in plasma during simultaneous COMT inhibition with entacapone. Theoretical pharmacokinetic simulations suggested that the plasma profile of oral IR levodopa can be even further improved by optimizing AADC and COMT inhibition.






Similar content being viewed by others
References
Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. 2020;27(1):27–42. https://doi.org/10.1111/ene.13914.
Hirsch EC. Biochemistry of Parkinson’s disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994;9(1–3):135–42. https://doi.org/10.1007/BF02816113.
Barbosa ER, Limongi JC, Cummings JL. Parkinson’s disease. Psychiatr Clin North Am. 1997;20(4):769–90. https://doi.org/10.1016/S0193-953X(05)70344-0.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397(10291):2284–303. https://doi.org/10.1016/S0140-6736(21)00218-X.
Prasad EM, Hung SY. Current therapies in clinical trials of Parkinson’s disease: a 2021 update. Pharmaceuticals (Basel). 2021;14(8):717. https://doi.org/10.3390/ph14080717.
Titova N, Levin O, Katunina E, Ray CK. “Levodopa Phobia”: a review of a not uncommon and consequential phenomenon. NPJ Parkinsons Dis. 2018;4:31. https://doi.org/10.1038/s41531-018-0067-z.
Chaudhuri KR, Jenner P, Antonini A. Should there be less emphasis on levodopa-induced dyskinesia in Parkinson’s disease? Mov Disord. 2019;34(6):816–9. https://doi.org/10.1002/mds.27691.
Espay AJ, Morgante F, Merola A, Fasano A, Marsili L, Fox SH, et al. Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol. 2018;84(6):797–811. https://doi.org/10.1002/ana.25364.
Gupta HV, Lyons KE, Wachter N, Pahwa R. Long term response to levodopa in Parkinson’s disease. J Parkinsons Dis. 2019;9(3):525–9. https://doi.org/10.3233/JPD-191633.
LeWitt PA, Giladi N, Navon N. Pharmacokinetics and efficacy of a novel formulation of carbidopa-levodopa (Accordion Pill®) in Parkinson’s disease. Parkinsonism Relat Disord. 2019;65:131–8. https://doi.org/10.1016/j.parkreldis.2019.05.032.
Tambasco N, Romoli M, Calabresi P. Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. 2018;16(8):1239–52. https://doi.org/10.2174/1570159X15666170510143821.
Nutt JG, Fellman JH. Pharmacokinetics of levodopa. Clin Neuropharmacol. 1984;7(1):35–49. https://doi.org/10.1097/00002826-198403000-00002.
Parkinson Study Group. Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Ann Neurol. 1997;42(5):747–55. https://doi.org/10.1002/ana.410420511.
Rinne UK, Larsen JP, Siden A, Worm-Petersen J. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group Neurology. 1998;51(5):1309–14. https://doi.org/10.1212/wnl.51.5.1309.
Waters CH, Kurth M, Bailey P, Shulman LM, LeWitt P, Dorflinger E, et al. Tolcapone in stable Parkinson’s disease: efficacy and safety of long-term treatment. The Tolcapone Stable Study Group Neurology. 1997;49(3):665–71. https://doi.org/10.1212/wnl.49.3.665.
Ferreira JJ, Lees A, Rocha JF, Poewe W, Rascol O, Soares-da-Silva P. Long-term efficacy of opicapone in fluctuating Parkinson's disease patients: a pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol. 2019 ;26(7):953-960. https://doi.org/10.1111/ene.13914
Robertson DR, Wood ND, Everest H, Monks K, Waller DG, Renwick AG, et al. The effect of age on the pharmacokinetics of levodopa administered alone and in the presence of carbidopa. Br J Clin Pharmacol. 1989;28(1):61–9. https://doi.org/10.1111/j.1365-2125.1989.tb03506.x.
Schultz E. Catechol-O-methyltransferase and aromatic L-amino acid decarboxylase activities in human gastrointestinal tissues. Life Sci. 1991;49(10):721–5. https://doi.org/10.1016/0024-3205(91)90104-j.
Novartis. Comtan drug label. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020796s15lbl.pdf. Accessed 11 July 2022.
Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34(13):4202–10. https://doi.org/10.1021/bi00013a008.
Goodall M, Alton H. Metabolism of 3,4-dihydroxyphenylalanine (L-dopa) in human subjects. Biochem Pharmacol. 1972;21(17):2401–8. https://doi.org/10.1016/0006-2952(72)90392-9.
Trenkwalder C, Kuoppamaki M, Vahteristo M, Muller T, Ellmen J. Increased dose of carbidopa with levodopa and entacapone improves “off” time in a randomized trial. Neurology. 2019;92(13):e1487–96. https://doi.org/10.1212/WNL.0000000000007173.
van Rumund A, Pavelka L, Esselink RAJ, Geurtz BPM, Wevers RA, Mollenhauer B, et al. Peripheral decarboxylase inhibitors paradoxically induce aromatic L-amino acid decarboxylase. NPJ Parkinsons Dis. 2021;7(1):29. https://doi.org/10.1038/s41531-021-00172-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Acknowledgements
The authors thank the participants, investigators, trial-site staff, and Orion Pharma employees involved in the studies. Hanna Wartiovaara is acknowledged for the supervision of the bioanalytical work. Mikko Koskinen is acknowledged for reviewing the manuscript.
Author contributions
Participated in clinical study design and study conduct: JT, MV, JE, JK and JR. Performed data analysis and modelling: JT, NS, MV and MY. All authors contributed to the writing of the manuscript and approved the final version for publication.
Funding
The clinical studies were sponsored by Orion Corporation, Orion Pharma. No funding was received for the preparation of this article.
Availability of data and material
The study data are available from the corresponding author upon reasonable request.
Code availability
Upon reasonable request.
Conflict of interest
Johanna Tuunainen, Mikko Vahteristo, Juha Ellmén, Mikko Kuoppamäki, and Juha Rouru were employees of Orion Corporation, Orion Pharma, Finland, at the time of study conduct. Mikko Vahteristo and Mikko Kuoppamäki own stock in Orion Corporation. Noora Sjöstedt and Marjo Yliperttula declare that they have no conflict of interest.
Ethics approval
All studies were conducted in accordance with the International Council for Harmonization Good Clinical Practice, the principles of the Declaration of Helsinki, and all applicable national regulations. The presented study was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa (250/13/03/00/2008). The studies used for pharmacokinetic model building were approved by the Manchester independent research ethics committee (ME1004/3097001) and the ethics committee of the Hospital District of Helsinki and Uusimaa.
Consent to participate
For all clinical studies described in the article, informed consent was obtained from the participants.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuunainen, J., Sjöstedt, N., Vahteristo, M. et al. Effect of Carbidopa Dose on Levodopa Pharmacokinetics With and Without Catechol-O-Methyltransferase Inhibition in Healthy Subjects. Eur J Drug Metab Pharmacokinet 48, 23–34 (2023). https://doi.org/10.1007/s13318-022-00800-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00800-w