Abstract
Background and Objectives
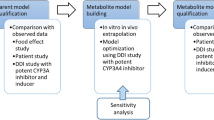
Due to health authority warnings and the recommended limited use of ketoconazole as a model inhibitor of cytochrome P450 (CYP) 3A4 in clinical drug–drug interaction (DDI) studies, there is a need to search for alternatives. Ritonavir is a strong inhibitor for CYP3A4/5-mediated DDIs and has been proposed as a suitable alternative to ketoconazole. It can also be used as a weak inhibitor for CYP2D6-mediated DDIs. Most of the currently available physiologically based pharmacokinetic (PBPK) inhibitor models developed for predicting DDIs use first-order absorption models, which do not mechanistically capture the effect of formulations on the systemic exposure of the inhibitor. Thus, the main purpose of the current study was to verify the predictive performance of a mechanistic absorption and disposition model of ritonavir when it was applied to the inhibition of CYP2D6 and CYP3A4/5 by ritonavir.
Methods
A PBPK model that incorporates formulation characteristics and enzyme kinetic parameters for post-absorptive pharmacokinetic processes of ritonavir was constructed. Key absorption-related parameters in the model were determined using mechanistic modelling of in vitro biopharmaceutics experiments. The model was verified for systemic exposure and DDI risk assessment using clinical observations from 13 and 18 studies, respectively.
Results
Maximal inhibition of hepatic (3.53% of the activity remaining) and gut (5.16% of the activity remaining) CYP3A4 activity was observed when ritonavir was orally administered in doses of 100 mg or higher. The PBPK model accurately described the concentrations of ritonavir in the different simulated studies. The prediction accuracy for maximum concentration (Cmax) and area under the plasma concentration versus time curve (AUC) were assessed. The bias (average fold error, AFE) for the prediction of Cmax and AUC was 0.92 and 1.06, respectively, and the precision (absolute average fold error, AAFE) was 1.29 and 1.23, respectively. The PBPK model predictions for all Cmax and AUC ratios when ritonavir was used as an inhibitor of CYP metabolism fell within twofold of the clinical observations. The prediction accuracy for Cmax and AUC ratios had a bias (AFE) of 0.85 and 0.99, respectively, and a precision (AAFE) of 1.21 and 1.33, respectively.
Conclusions
The current model, which incorporates formulation characteristics and mechanistic disposition parameters, can be used to assess the DDI potential of CYP3A4/5 and CYP2D6 substrates administered with a twice-daily dose of 100 mg of ritonavir for 14 days.



Similar content being viewed by others
References
Kuemmel C, Yang Y, Zhang X, Florian J, Zhu H, Tegenge M, et al. Consideration of a credibility assessment framework in model-informed drug development: potential application to physiologically-based pharmacokinetic modeling and simulation. CPT Pharmacomet Syst Pharmacol. 2020;9:21–8.
Huang SM, Abernethy DR, Wang Y, Zhao P, Zineh I. The utility of modeling and simulation in drug development and regulatory review. J Pharm Sci. 2013;102:2912–23.
Zineh I, Abernethy D, Hop C, Bello A, McClellan MB, Daniel GW, et al. Improving the tools of clinical pharmacology: Goals for 2017 and beyond. Clin Pharmacol Ther. 2017;101:22–4.
Grimstein M, Yang Y, Zhang X, Grillo J, Huang SM, Zineh I, et al. Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. Food and Drug Administration’s Office of Clinical Pharmacology. J Pharm Sci. 2019;108:21–5.
FDA. Drug development and drug interactions: table of substrates, inhibitors and inducers. 2020. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers. Accessed 9 Feb 2021.
Koudriakova T, Iatsimirskaia E, Utkin I, Gangl E, Vouros P, Storozhuk E, et al. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: mechanism-based inactivation of cytochrome P4503A by ritonavir. Drug Metab Dispos. 1998;26:552–61.
Shebley M, Liu J, Kavetskaia O, Sydor J, de Morais SM, Fischer V, et al. Mechanisms and predictions of drug-drug interactions of the hepatitis C virus three direct-acting antiviral regimen: paritaprevir/ritonavir, ombitasvir, and dasabuvir. Drug Metab Dispos. 2017;45:755–64.
Umehara KI, Huth F, Won CS, Heimbach T, He H. Verification of a physiologically based pharmacokinetic model of ritonavir to estimate drug-drug interaction potential of CYP3A4 substrates. Biopharm Drug Dispos. 2018;39:152–63.
Mathias AA, West S, Hui J, Kearney BP. Dose-response of ritonavir on hepatic CYP3A activity and elvitegravir oral exposure. Clin Pharmacol Ther. 2009;85:64–70.
Arora S, Pansari A, Kilford P, Jamei M, Gardner I, Turner DB. Biopharmaceutic in vitro in vivo extrapolation (IVIV_E) informed physiologically-based pharmacokinetic model of ritonavir Norvir tablet absorption in humans under fasted and fed state conditions. Mol Pharm. 2020;17:2329–44.
FDA. Application number: 20-945. Clinical pharmacology and biopharmaceutics review(s). Silver Spring, MD: Center for Drug Evaluation and Research; 1997.
Liu P, Foster G, Gandelman K, LaBadie RR, Allison MJ, Gutierrez MJ, et al. Steady-state pharmacokinetic and safety profiles of voriconazole and ritonavir in healthy male subjects. Antimicrob Agents Chemother. 2007;51:3617–26.
Wang J, Flanagan DR. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory J Pharm Sci. 1999;88:731–8.
Sugano K. Theoretical investigation of passive intestinal membrane permeability using Monte Carlo method to generate drug-like molecule population. Int J Pharm. 2009;373:55–61.
Pade D, Jamei M, Rostami-Hodjegan A, Turner DB. Application of the MechPeff model to predict passive effective intestinal permeability in the different regions of the rodent small intestine and colon. Biopharm Drug Dispos. 2017;38:94–114.
Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:1070–8.
Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–31.
von Moltke LL, Greenblatt DJ, Duan SX, Daily JP, Harmatz JS, Shader RI. Inhibition of desipramine hydroxylation (cytochrome P450–2D6) in vitro by quinidine and by viral protease inhibitors: relation to drug interactions in vivo. J Pharm Sci. 1998;87:1184–9.
Bertz R, Cao G, Cavanaugh J, Hsu A, Granneman G, Leonard J. Effect of ritonavir on the pharmacokinetics of desipramine. In: 11th International Conference on AIDS; 1996 July 7–12; Vancouver, Canada. p. 7–12.
Penzak SR, Shen JM, Alfaro RM, Remaley AT, Natarajan V, Falloon J. Ritonavir decreases the nonrenal clearance of digoxin in healthy volunteers with known MDR1 genotypes. Ther Drug Monit. 2004;26:322–30.
Fahmi OA, Maurer TS, Kish M, Cardenas E, Boldt S, Nettleton D. A combined model for predicting CYP3A4 clinical net drug-drug interaction based on CYP3A4 inhibition, inactivation, and induction determined in vitro. Drug Metab Dispos. 2008;36:1698–708.
Ieiri I, Tsunemitsu S, Maeda K, Ando Y, Izumi N, Kimura M, et al. Mechanisms of pharmacokinetic enhancement between ritonavir and saquinavir; micro/small dosing tests using midazolam (CYP3A4), fexofenadine (p-glycoprotein), and pravastatin (OATP1B1) as probe drugs. J Clin Pharmacol. 2013;53:654–61.
Shimizu H, Yoshida K, Nakada T, Kojima K, Ogasawara A, Nakamaru Y, et al. Prediction of human distribution volumes of compounds in various elimination phases using physiologically based pharmacokinetic modeling and experimental pharmacokinetics in animals. Drug Metab Dispos. 2019;47:114–23.
Guest EJ, Aarons L, Houston JB, Rostami-Hodjegan A, Galetin A. Critique of the two-fold measure of prediction success for ratios: application for the assessment of drug-drug interactions. Drug Metab Dispos. 2011;39:170–3.
Darwich AS, von Moltke L. The impact of formulation, delivery, and dosing regimen on the risk of drug-drug interactions. Clin Pharmacol Ther. 2019;105:1329–31.
Taskar KS, Pilla Reddy V, Burt H, Posada MM, Varma M, Zheng M, et al. Physiologically-based pharmacokinetic models for evaluating membrane transporter mediated drug-drug interactions: current capabilities, case studies, future opportunities, and recommendations. Clin Pharmacol Ther. 2019;107:1082–115.
Liu B, Crewe HK, Ozdemir M, Rowland Yeo K, Tucker G, Rostami-Hodjegan A. The absorption kinetics of ketoconazole plays a major role in explaining the reported variability in the level of interaction with midazolam: interplay between formulation and inhibition of gut wall and liver metabolism. Biopharm Drug Dispos. 2017;38:260–70.
Pathak SM, Ruff A, Kostewicz ES, Patel N, Turner DB, Jamei M. Model-based analysis of biopharmaceutic experiments to improve mechanistic oral absorption modeling: an integrated in vitro in vivo extrapolation perspective using ketoconazole as a model drug. Mol Pharm. 2017;14:4305–20.
Chen Y, Ma F, Jones NS, Yoshida K, Chiang PC, Durk MR, et al. Physiologically-based pharmacokinetic model-informed drug development for fenebrutinib: understanding complex drug-drug interactions. CPT Pharmacometrics Syst Pharmacol. 2020;9:332–41.
Ellenberger DJ, Miller DA, Kucera SU, Williams RO 3rd. Generation of a weakly acidic amorphous solid dispersion of the weak base ritonavir with equivalent in vitro and in vivo performance to Norvir tablet. AAPS PharmSciTech. 2018;19:1985–97.
Ouellet D, Hsu A, Granneman GR, Carlson G, Cavanaugh J, Guenther H, et al. Pharmacokinetic interaction between ritonavir and clarithromycin. Clin Pharmacol Ther. 1998;64:355–62.
Kirby BJ, Collier AC, Kharasch ED, Dixit V, Desai P, Whittington D, et al. Complex drug interactions of HIV protease inhibitors 2: in vivo induction and in vitro to in vivo correlation of induction of cytochrome P450 1A2, 2B6, and 2C9 by ritonavir or nelfinavir. Drug Metab Dispos. 2011;39:2329–37.
Eichbaum C, Cortese M, Blank A, Burhenne J, Mikus G. Concentration effect relationship of CYP3A inhibition by ritonavir in humans. Eur J Clin Pharmacol. 2013;69:1795–800.
Law D, Krill SL, Schmitt EA, Fort JJ, Qiu Y, Wang W, et al. Physicochemical considerations in the preparation of amorphous ritonavir-poly(ethylene glycol) 8000 solid dispersions. J Pharm Sci. 2001;90:1015–25.
Xu H, Vela S, Shi Y, Marroum P, Gao P. In vitro characterization of ritonavir drug products and correlation to human in vivo performance. Mol Pharm. 2017;14:3801–14.
Indulkar AS, Gao Y, Raina SA, Zhang GGZ, Taylor LS. Crystallization from supersaturated solutions: role of lecithin and composite simulated intestinal fluid. Pharm Res. 2018;35:158.
Zhang C, Denti P, Decloedt E, Maartens G, Karlsson MO, Simonsson US, et al. Model-based approach to dose optimization of lopinavir/ritonavir when co-administered with rifampicin. Br J Clin Pharmacol. 2012;73:758–67.
Hsu A, Granneman GR, Witt G, Locke C, Denissen J, Molla A, et al. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905.
Ng J, Klein CE, Chui YL, Awni WM, Morris JB, Podsadecki TJ, et al. The effect of food on ritonavir bioavailability following administration of ritonavir 100 mg film-coated tablet in healthy adult subjects. J Int AIDS Soc. 2008;11.
Teng S, Potvin D, Mamputu JC, Vincent G, Zoltowska M, Morin J, et al. Impact of tesamorelin, a growth hormone-releasing factor (GRF) analogue, on the pharmacokinetics of simvastatin and ritonavir in healthy volunteers. Clin Pharmacol Drug Dev. 2013;2:237–45.
Brennan BJ, Moreira SA, Morcos PN, et al. Pharmacokinetics of a three-way drug interaction between danoprevir, ritonavir and the organic anion transporting polypeptide (OATP) inhibitor ciclosporin. Clin Pharmacokinet. 2013;52(9):805–13.
Brennan BJ, Poirier A, Moreira S, Morcos PN, Goelzer P, Portmann R, et al. Characterization of the transmembrane transport and absolute bioavailability of the HCV protease inhibitor danoprevir. Clin Pharmacokinet. 2015;54:537–49.
Morris CA, Lopez-Lazaro L, Jung D, Methaneethorn J, Duparc S, Borghini-Fuhrer I, et al. Drug-drug interaction analysis of pyronaridine/artesunate and ritonavir in healthy volunteers. Am J Trop Med Hyg. 2012;86:489–95.
Morcos PN, Moreira SA, Navarro MT, Bech N, Quatkemeyer A, Smith PF, et al. Effect of meal and antisecretory agents on the pharmacokinetics of danoprevir/ritonavir in healthy volunteers. J Pharm Pharmacol. 2014;66:23–31.
Aarnoutse RE, Kleinnijenhuis J, Koopmans PP, Touw DJ, Wieling J, Hekster YA, et al. Effect of low-dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin Pharmacol Ther. 2005;78:664–74.
Ancrenaz V, Deglon J, Samer C, Staub C, Dayer P, Daali Y, et al. Pharmacokinetic interaction between prasugrel and ritonavir in healthy volunteers. Basic Clin Pharmacol Toxicol. 2013;112:132–7.
Greenblatt DJ, Peters DE, Oleson LE, Harmatz JS, MacNab MW, Berkowitz N, et al. Inhibition of oral midazolam clearance by boosting doses of ritonavir, and by 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an experimental catalytic mimic of glutathione oxidase. Br J Clin Pharmacol. 2009;68:920–7.
Mathias AA, German P, Murray BP, Wei L, Jain A, West S, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010;87:322–9.
Greenblatt DJ, von Moltke LL, Harmatz JS, Durol AL, Daily JP, Graf JA, et al. Alprazolam-ritonavir interaction: implications for product labeling. Clin Pharmacol Ther. 2000;67:335–41.
Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex drug interactions of the HIV protease inhibitors 3: effect of simultaneous or staggered dosing of digoxin and ritonavir, nelfinavir, rifampin, or bupropion. Drug Metab Dispos. 2012;40:610–6.
Acknowledgements
The authors would like to thank Eleanor Savill for assistance with submitting the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The activities of Certara UK, Simcyp Division are supported by the Simcyp Consortium of pharmaceutical companies. No other source of funding was used for the work.
Conflict of interest
At the time of the work, Sumit Arora was employed by Certara UK Limited and may hold shares in the company. At the time of the work, Peter Kilford was employed by Certara UK Limited and may hold shares in the company. Amita Pansari is employed by Certara UK Limited and may hold shares in the company. David Turner is employed by Certara UK Limited and may hold shares in the company. Iain Gardner is employed by Certara UK Limited and may hold shares in the company. Masoud Jamei is employed by Certara UK Limited and may hold shares in the company.
Author Contributions
SA, PK, AP, DT, IG, and MJ wrote the manuscript; SA, AP, and PK designed the research; SA, AP, PK, and IG performed the research; SA, AP, PK, and IG analysed the data.
Ethics approval
This was a simulation study and did not involve any clinical studies, and therefore ethics approval was not needed.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The workspaces used to run the simulation are available from the corresponding author on reasonable request.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arora, S., Pansari, A., Kilford, P.J. et al. A Mechanistic Absorption and Disposition Model of Ritonavir to Predict Exposure and Drug–Drug Interaction Potential of CYP3A4/5 and CYP2D6 Substrates. Eur J Drug Metab Pharmacokinet 47, 483–495 (2022). https://doi.org/10.1007/s13318-022-00765-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00765-w