Abstract
Background and Objective
The antitumor activity and toxicity of doxorubicin are potentiated and attenuated by calcitriol, respectively. Potentially, calcitriol can be combined with doxorubicin for clinical benefit in chemotherapy. To gain insight into the interaction between doxorubicin and calcitriol, proposed for combined use in cancer treatment, we studied calcitriol's effect on the plasma pharmacokinetics, tissue distribution and excretion of doxorubicin in female and male mice.
Methods
The control and calcitriol-treated groups, including an equal number of both sexes, received corn oil and calcitriol (2.5 μg/kg), respectively, intraperitoneally every other day for 8 days. At day 9, doxorubicin was administered intraperitoneally at a 6 mg/kg dose to each group. Doxorubicin concentrations in biologic specimens were determined by a high-performance liquid chromatographic-ultraviolet detector and analyzed using a non-compartmental model.
Results
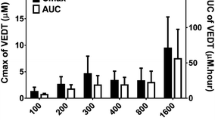
The plasma pharmacokinetics of doxorubicin were similar in the control and calcitriol-treated groups. While calcitriol did not alter the area under the plasma concentration-time curves (AUCs) and peak concentrations (Cmax) of doxorubicin in the small intestine and testis, it significantly reduced the AUCs and Cmax of doxorubicin in the lung, kidney, spleen, liver, stomach and ovaries. However, calcitriol increased the AUCs and Cmax of doxorubicin in the heart of females, brain of males and duodenum content and vitreous humor of female and male mice. The percent cumulative urine and fecal amounts of doxorubicin in calcitriol-treated mice were higher at 89.23% and 29.37% for female mice and 118.57% and 41.65% for male mice than those in the control mice, respectively.
Conclusions
The tissue concentrations and excretion of doxorubicin in both female and male mice are influenced by calcitriol without changes in the plasma pharmacokinetics. The results from this study can provide insights to help obtain the optimal drug combination effects of doxorubicin with calcitriol in cancer treatment.




Similar content being viewed by others
References
Kumar A, Gautam B, Dubey C, Tripathi PK. A review: role of doxorubicin in treatment of cancer. Int J Pharm Sci Rev Res. 2014;5(10):4117–288.
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–85.
Kaye S, Merry S. Tumour cell resistance to anthracyclines—a review. Cancer Chemother Pharmacol. 1985;14:96–103.
Sadzuka Y, Egawa Y, Sawanishi H, Miyamoto K, Sonobe T. Effects of xanthine derivatives on the influx and efflux of doxorubicin in P388 and DOX-resistant P388 leukemia cells. Toxicol Lett. 2002;135:137–44.
Gustafson DL, Merz AL, Long ME. Pharmacokinetics of combined doxorubicin and paclitaxel in mice. Cancer Lett. 2005;220:161–9.
Hudachek SF, Gustafson DL. Coadministration of lapatinib increases exposure to docetaxel but not doxorubicin in the small intestine of mice. Anti-Cancer Drug. 2013;24:958–68.
Kim TH, Shin YJ, Won AJ, Lee BM, Choi WS, Jung JH, Chung HY, Kim HS. Resveratrol enhances chemosensitivity of doxorubicin in multidrug-resistant human breast cancer cells via increased cellular influx of doxorubicin. Biochim Biophys Acta. 2014;1840:615–25.
Fleet JC. Molecular actions of vitamin D contributing to cancer prevention. Mol Aspects Med. 2008;29:388–96.
Gabriel JR. Vitamin D. Actas Dermosifiliogr. 2010;101(9):739–41.
Johnson CS, Hershberger PA, Bernardi RJ, Mcguire TF, Trump DL. Vitamin D receptor: a potential target for interventıon. Urology. 2002;60(3):123–31.
Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80:1678–88.
Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev. 2007;7:685–700.
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;4:1–16.
Ben-Eltriki M, Deb S, Guns EST. Calcitriol in combination therapy for prostate cancer: pharmacokinetic and pharmacodynamic interactions. J Cancer. 2016;7(4):391–407.
Trump DL, Hershberger PA, Bernardi RJ, Ahmed S, Muindi J, Fakih M, Yu WD, Johnson SC. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90:519–26.
Yan M, Nuriding H. Reversal effect of vitamin D on different multidrug-resistant cells. Genet Mol Res. 2014;13(3):6239–47.
Ping-ping H, Dong F, Miao C. 1,25-(OH)2D3 enhances the chemotherapeutic effect of doxorubicin on gastric carcinoma cells. Tumor. 2011;31(4):304–9.
Tsai TH, Lin CJ, Hang CL, Chen WY. Calcitriol attenuates doxorubicin-induced cardiac dysfunction and inhibits endothelial-to-mesenchymal transition in mice. Cells. 2019;8(8):E865. https://doi.org/10.3390/cells8080865.
Kim JE, Cho HJ, Kim JS, Shim CK, Chung SJ, Oak MH, Yoon IS, Kim DD. The limited intestinal absorption via paracellular pathway is responsible for the low oral bioavailability of doxorubicin. Xenobiotica. 2013;43(7):579–91.
Durmus S, Naik J, Buil L, Wagenaar E, Tellingen OV, Schinkel AH. In vivo disposition of doxorubicin is affected by mouse Oatp1a/1b and human OATP1A/1B transporters. Int J Cancer. 2014;135:1700–10.
Lee HH, Leake BF, Kim RB, Ho RH. Contribution of organic anion-transporting polypeptides 1A/1B to doxorubicin uptake and clearance. Mol Pharmacol. 2016;24:1–36.
Nishida S, Ozeki J, Makishima M. Modulation of bile acid metabolism by 1α-hydroxyvitamin D3 administration in mice. J Pharmacol Exp Ther. 2009;37:2037–44.
Chow ECY, Sun H, Khan AA, Groothuis GMM, Pang KS. Effects of 1α, 25-dihydroxyvitamin d3 on transporters and enzymes of the rat ıntestine and kidney in vivo. Biopharm Drug Dispos. 2010;31:91–108.
Eloranta JJ, Hiller C, Juttner M, Kullak-Ublick GA. The SLCO1A2 gene, encoding human organic anion-transporting polypeptide 1A2, is transactivated by the vitamin d receptor. J Pharmacol Exp Ther. 2012;82:37–46.
Suzuki T, Zhao YL, Nadai M, Naruhashi K, Shimizu A, Takagi K, Takagi K, Hasegawa T. Gender-related differences in expression and function of hepatic P-glycoprotein and multidrug resistance-associated protein (Mrp2) in rats. Life Sci. 2006;79:455–61.
Bebawy M, Chetty M. Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome. Curr Drug Metab. 2009;10:322–8.
Stute P, Reichenbach A, Szuwart T, Kiesel L, Götte M. Impact of testosterone on the expression of organic anion transporting polypeptides (OATP-1A2, OATP-2B1, OATP-3A1) in malignant and non-malignant human breast cells in vitro. Maturitas. 2012;71:376–84.
Ahmad M, Usman M, Madni A, Zubair M, Zaman Q, Qureshi MS, Munir A, Ahmad M, Mahmood A. A fast and simple HPLC-UV method for simultaneous determination of three anti-cancer agents in plasma of breast cancer patients and its application to clinical pharmacokinetics. Afr J Pharm Pharmaco. 2011;5(7):915–22.
Sambasivam G, Shewade DG, Dubashi B, Sundaram R. A simple and rapid method for simultaneous quantification of doxorubicin, cpirubicin, cyclophosphamide and 5-fluorouracil in human plasma by LCMS/MS. World J Pharm Res. 2016;5(10):747–57.
FDA. Guidance for industry bioanalytical method validation. U.S. department of health and human services, food and drug administration, center for drug evaluation and research (CDER), Center for Veterinary Medicine (CVM). 2001. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed 26 Feb 2020.
EMA. Guideline on bioanalytical method validation. Committee for Medicinal Products for Human Use (CHMP), European Medecines Agency (EMA). 2011. https://www.ema.europa.eu/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf. Accessed 26 Feb 2020.
Gibaldi M, Perrier D. Pharmacokinetics. 3rd ed. New York: Marcel Dekker Inc; 1982. p. 145–198.
Corum O, Er A, Corum DD, Atik O, Uney K. Pharmacokinetics and bioavailability of ceftriaxone in brown trout (Salmo T trutta fario) after intravenous and intramuscular administration. Aquaculture. 2019;500:272–7.
Colombo T, Zucchetti M, D'Incalci M. Cyclosporin A markedly changes the distribution of doxorubicin in mice and rats. J Pharmacol Exp Ther. 1994;269(1):22–7.
Asperen JV, Tellingen OV, Tijssen F, Schinkel AH, Beijnen JH. Increased accumulation of doxorubicin and doxorubicinol in cardiac tissue of mice lacking mdr1a P-glycoprotein. Br J Cancer. 1999;79(1):108–13.
Schrijvers D. Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Clin Pharmacokinet. 2003;42(9):779–91.
Chow ECY, Sondervan M, Jin C, Groothuıs GMM, Pang KS. Comparative effects of doxercalciferol (1α-hydroxyvitamin D2) versus calcitriol (1α,25-dihydroxyvitamin D3) on the expression of transporters and enzymes in the rat in vivo. J Pharm Sci. 2011;100:1594–604.
Tangpong J, Miriyala S, Noel T, Sinthupibulyakit C, Jungsuwadee P, Clairb DK. St. Doxorubıcın-ınduced central nervous system toxıcıty and protectıon by xanthone derıvatıve of garcınıa mangostana. Euroscience. 2011;23(175):292–9.
Pugazhendhia A, Edison TNJI, Velmuruganc BK, Jacob JA, Karuppusamy I. Toxicity of doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018;200:26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was summarized from a PhD thesis and supported by The Coordination of Scientific Research Projects, University of Selcuk, Turkey (project no. 16202023).
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All study protocols were approved by Selcuk University Experimental Medical Application and Research Center's Ethics Committee (2016/21, Konya, Turkey). Animal care was carried out as per all institutional and national guidelines.
Rights and permissions
About this article
Cite this article
Durna Corum, D., Uney, K. Gender Differences in the Effect of Calcitriol on the Body Disposition and Excretion of Doxorubicin in Mice. Eur J Drug Metab Pharmacokinet 45, 653–664 (2020). https://doi.org/10.1007/s13318-020-00632-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00632-6