Abstract
Backgrounds and Objectives
The use of local antibiotic delivery vehicles is common in the management of biofilm-related infections as they provide high concentrations of local antibiotics while simultaneously avoiding complications from systemic toxicity. We present a 100% pure synthetic calcium sulfate hemi-hydrate mixed with 240 mg tobramycin and 500 mg vancomycin per 10 cc mixture for use in revision surgeries of periprosthetic joint infections (PJIs). The purified carrier demonstrates bioabsorbablity, promotion of bone growth, a physiologically favorable pH, and hydrophilicity. These unique properties may alleviate persistent postoperative wound drainage seen in patients with PJI. Our questions consist of two parts: (1) does the novel calcium sulfate carrier provide therapeutic concentrations of antibiotic locally that can kill biofilm related infections? (2) Are serum concentrations of antibiotic significant to cause concern for systemic toxicity?
Methods
To address these questions, we assayed the elution of antibiotic concentrations obtained from surgical drains and serum among 50 patients in the first 5 postoperative days.
Results
The elution of vancomycin and tobramycin was greatest on day 1 compared with those concentrations obtained on days 2, 3, 4, and 5; serum concentrations were largely undetectable. Our findings demonstrate that this calcium sulfate preparation provides therapeutic delivery of vancomycin and tobramycin locally at log 2–3 above the minimum inhibitory concentration (MIC), while avoiding toxic serum concentrations.
Conclusions
When used in one-stage revision arthroplasties, the bioabsorbable, purified carrier delivers high concentrations of antibiotic while avoiding systemic toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Local antibiotic delivery can effectively reduce biofilm formation |
Synthetic calcium sulfate's unique properties optimize antibiotic delivery |
Calcium sulfate carries therapeutic antibiotic levels without systemic toxicity |
1 Introduction
Chronic infection presents a serious complication following the surgical implantation of total joint prosthetics. Once a patient becomes contaminated, bacteria adhere to surfaces of implanted components and form colonies (biofilm) that enter a state of chronic latency [1,2,3,4]. Bacteria entering into this sessile form may produce biofilm matrices (glycocalyx); they proliferate, exchange genetic information, and proliferate up to a point and stop (quorum sensing), leading to asymptomatic infections [5, 6]. With biofilm development, pathogen genomes are altered through cell-to-cell signaling pathways, becoming phenotypically distinct from planktonic forms [6]. This change allows sessile forms to develop recalcitrance to immune regulatory mechanisms and antimicrobial therapy, causing much concern over the management of chronic infection [5, 7,8,9]. Ideally, drugs used in treating biofilm-mediated infections should be capable of disrupting the bacteria’s structure [5, 7, 10, 11]. However, parenterally administered antibiotics treat planktonic bacterial infections with more success than the sessile forms [5, 11].
In response, surgeons employ local antibiotic carriers as an adjunct to one- and two-stage revision protocols for infected total joint prosthetics [12,13,14,15,16,17]. The use of local delivery mechanisms provides high concentrations of drugs at the infection site with minimal serum concentrations [12, 18,19,20,21]. Furthermore, maintaining local antibiotic concentrations many times above the minimum inhibitory concentration (MIC) discourages the development of bacterial resistance that prolonged exposure to antibiotics below inhibitory concentrations may cause [20]. Currently, the use of antibiotic-impregnated poly-methyl-methacrylate (PMMA) spacers sets the standard for antibiotic therapy in two-stage revisions of septic joints [5, 16, 17, 21,22,23,24]. As PMMA spacers are not bioabsorbable, additional surgery becomes necessary for removal, thus rendering them inadequate as soft tissue fillers in one-stage treatment of osteomyelitis [12, 16, 17]. Moreover, the surface roughness of PMMA may potentiate bacterial adhesion, possibly leading to secondary infection [18, 25,26,27,28]. Finally, the use of PMMA may be recovered in the serum [16, 17], correlating with the observation of sustained antibiotic serum concentrations that may result in allergic reactions or complications [16, 17, 29].
As an alternative, calcium sulfate carriers offer multiple significant advantages over PMMA. These advantages include the reabsorption of calcium sulfate within weeks, the carrier releases its entire antibiotic load when still visualized by x-ray, mixture with heat-sensitive antibiotics remains unproblematic because of minimal heat production on curing, the pelletized carrier obliterates dead space following debridement of bone and soft tissue, and calcium sulfate serves as a potential synthetic osteoconductive bone substitute when placed in the bone [30,31,32,33,34].
These advantages make the use of a calcium sulfate vehicle intriguing for application in several scenarios, including abscess or soft tissue infection treatment, prophylaxis in high-risk patients, use with physiologic constructs in one-stage procedures for osteomyelitis, and as an adjunct to antibiotic-loaded bone cement. Preparation of calcium sulfate beads provides a stable platform for the delivery antibiotics; however, problems associated with the use of calcium sulfate involve complications with persistent postoperative wound drainage [35,36,37,38,39,40]. We feel this drainage may be due to specific physical properties of gypsum-mined calcium sulfate preparations, including its hydrophobicity, acidic pH, and impurity due to the presence of trace minerals as seen by Fourier transform infrared (FTIR) analysis. FTIR analysis involves transmitting infrared radiation through a material with a detector on the other side of the material reading the resulting spectrum of infrared radiation. The spectrum picked up by the detector then represents the chemical make-up of the material that is unique to materials only with that specific molecular structure, making it a useful tool in analyzing materials for quality [41].
This study examines clinical elution profiles of a purified calcium sulfate preparation in contrast to other less pure forms. Thus, we ask the following: (1) does this calcium sulfate preparation provide local delivery of antibiotics at concentrations exceeding MIC of common infecting pathogens? (2) Will serum concentrations of antibiotics remain at safe concentrations?
2 Patients and Methods
For the purposes of this study, we chose Stimulan® (Biocomposites, Keele Science Park, Staffordshire, UK) as the purified antibiotic carrier. Since a broad-spectrum antibiotic preparation provides coverage against gram-positive and -negative species with few resistant bacterial strains, we selected vancomycin and tobramycin to treat gram-positive and -negative bacteria, respectively [42]. These antibiotics also act as an effective combination due to their synergistic bactericidal potential in treating serious infections involving Staphylococcus aureus and other pathogens [20].
Calcium sulfate hemi-hydrate powder reacts with water provided by liquid tobramycin and sets according to the following reaction:
The addition of antibiotics affects this setting reaction. Tobramycin, in liquid form, accelerates setting rates as opposed to the effect of vancomycin powder, with higher additions having a greater effect for delayed setting. See Table 1 for a comparison of preparation notes for liquid and powder forms of tobramycin with the carrier.
The mixing of calcium sulfate with the antibiotics is performed in the sterile field of the operating room intraoperatively. Tobramycin preparation and dose were prepared identically for each patient, according to Table 1. Vancomycin was also prepared identically for each patient, using a 500 mg per 10 cc pellet mixture. For surgical placement, the calcium sulfate/antibiotic paste is pressed into a pelletizing mold (supplied with the product, Fig. 1) to produce beads of different dimensions. The paste is allowed to set within the mold, and the resulting beads are removed by flexing the mold over a sterile container. The beads remain covered in the container until implantation.
The first 50 patients that consented to the procedure were included (Table 2). There was no differentiation based on gender or age. Patients with a GFR > 60 and a cleared preoperative evaluation were viable candidates. Both serum and local drain samples were collected every morning at 0600 and taken immediately to the laboratory for same-day analysis. Serum samples were collected in red-top tubes with 3-ml aliquots. The local drain samples were collected in sterile glass tubes with 5-ml aliquots.
Upon completion of surgical debridement and implantation of prosthetic components, the calcium sulfate pellets are applied to the wound for dead space management with high concentrations of antibiotic bleaching. Patients are then fitted with a drain prior to wound closure for collection of the local exudates. In the 5 postoperative days, serum and drain samples are assayed with fluorescence polarization immunoassay (FPIA) with the Abbott Architect C8000 Chemistry Analyzer to evaluate the eluting characteristics of the Stimulan® carrier impregnated with vancomycin and tobramycin at the above-mentioned concentrations. As FPIA executes a single step to assay samples, it offers high-throughput screening of large numbers of samples.
2.1 Bioanalytical Assay
All samples were tested via the Abbott Architect C8000 Chemistry Analyzer in the same manner. Upon collection and delivery to the laboratory, the protocol provided by the Abbott User Manual was followed [43]. The internal standard was sterile water. The upper and lower limits of quantification were 15 and 5 times the concentration of the internal standard, respectively. The laboratory was in-house, Texas Health Presbyterian Dallas, and operated by board-certified pathologists and laboratory technicians who performed routine quality improvement/quality ssurance with the device.
3 Results
We analyzed 50 patients undergoing revision arthroplasty for infected total joints or major multiple revisions. Cases included 33 knees (1 bilateral), 15 hips, 1 elbow, and 1 shoulder from 22 females and 28 males. Average patient age was 61 years (range 13–82). None of the patients experienced relapse of infection or wound complications secondary to persistent wound drainage.
We evaluated local antibiotic concentrations to determine whether the concentrations contained in the eluent exceeded the MIC of common infecting pathogens and concentrations in the serum were also evaluated for all subjects [44,45,46,47]. Table 3 lists the results.
Mean values obtained from the local exudate demonstrated therapeutic concentrations of vancomycin and tobramycin in each of the 5 postoperative days. Concentrations of both antibiotics peaked on day 1 (averages: vancomycin: 265 µg/ml; tobramycin: 31 µg/ml, and ranges: vancomycin: 28.3–736.4; tobramycin: 6.4–97.2). Table 4 lists the change in mean values over time for each of the 50 subjects, and Figs. 2 and 3 illustrate the trend in mean value reduction over time.
We evaluated drain and blood serum concentrations of vancomycin and tobramycin on a standard hospital assay for these drugs in the 5 postoperative days. The assay reported antibiotic concentrations that were detected within specific concentration limits (vancomycin 2–400 µg/ml, tobramycin 0.5–20 µg/ml). Additional specific maximum assayable values for each antibiotic were recorded for a selection of the results. The majority of patients exhibited antibiotic blood serum concentrations below the lower concentration limits of the standard assay (vancomycin < 2 µg/ml, tobramycin < 0.5 µg/ml). However, a few patients exhibited detectable serum concentrations of antibiotics as follows: 11 (of the 50 patients) at day 1, 4 patients at day 2, and 3 patients at days 3 and 4. Table 5 lists the antibiotic serum concentrations for each patient.
We evaluated the purity of the Stimulan® calcium sulfate preparation when compared with a commonly used calcium sulfate carrier, with a Fourier transform infrared analysis (FTIR). The results of this comparison are provided in Fig. 4 and Table 6. The use of FTIR spectroscopy provides a ‘finger-print’ for characterization of a material. Broader peaks indicate an increasing presence of impurities in the chemical lattice.
4 Discussion
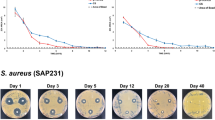
By controlling the source of infection in total joint prosthetics through the disruption of biofilm formation, the surgeon creates an acute wound milieu that helps activate host immune mechanisms for effective wound healing. This control may be accomplished by combining the strict adherence to wound management through radical debridement with the administration of a local antibiotic depot capable of eluting potent concentrations for killing exposed biofilm [48]. Antibiotic-laden pure calcium sulfate hemihydrate aids in treatment of infected avascular bone isolated from systemic antibiotics while avoiding complications of toxicity associated with parenteral administration. A mixture of broad-spectrum antibiotics perpetuates eradication of heterogeneous bacterial phenotypes. We prefer vancomycin and tobramycin for this purpose as the concentrations eluded locally kill biofilm-related infections as demonstrated in the center for the biofilm engineering drip reactor system [48]. The pure form of the calcium sulfate carrier distinguishes itself from other preparations by its higher purity, physiologic pH, and hydrophilic properties. This bead becomes soft after hydration, allowing for placement in joints without scratching the articular surfaces, and demonstrates complete dissolution within a few weeks as illustrated by the radiographic films in Fig. 5 [49].
Monitoring the concentrations of antibiotic eluate allows for confidence in their therapeutic benefits, which depend on sustained blood concentrations at a minimum concentration while local concentrations remain high for the duration of therapy. Furthermore, excessive serum concentrations of vancomycin and tobramycin must be avoided because high concentrations may result in serious side effects, specifically ototoxicity and nephrotoxicity. The purpose of our study was therefore twofold: to verify that concentrations of vancomycin and tobramycin eluted from calcium sulfate would still be capable of inhibiting bacterial growth; to determine whether the antibiotics eluted from the pellet would remain local without elevating serum concentrations, which may result in general toxicity.
Limitations of this study include the ceiling placed on assayable values of antibiotic concentrations (as determined by FPIA analysis), the duration of evaluation, and the comparison between different volumes placed into wounds. Despite the truncated measurements, this study still demonstrates expected local and systemic elution characteristics. Regarding the temporal limitations, we would have preferred continued measurement of the antibiotic concentrations. However, our decision to discontinue after 5 days was based on concern for contamination from prolonged placement of the drains. Some of the patients in which we observed detectable serum concentrations also had concurrent implantation of PMMA cement impregnated with vancomycin and/or tobramycin as part of the reconstructive surgery. This concurrent implantation of an additional antibiotic delivery system may be a contributory factor to the incidences of detectable serum concentrations. Furthermore, controlling for the patient population may have yielded different results. For example, GFR changes with age and gender; therefore, medication levels may be different based on the specific patient. Also, a comparative group with PMMA or a control was not measured. Inclusion of a comparative group would have improved the specificity of the results and provided more evidence for the superiority of the Stimulan beads. However, there is significant evidence of the inefficacy of the PMMA for antibiotic implantation [16,17,18]. Additionally, this study was not designed for comparison against another product, but to show that the Stimulan beads can produce antibiotic concentrations high enough to kill bacteria related to biofilm infections but not too high to be toxic to the patient.
The clinical data also had limitations due to inaccuracy of antibiotic concentrations in the eluent as a result of the standard laboratory assay used. The laboratory agency conducting the FPIA analysis established standard routine reporting of antibiotic concentrations within specific concentration limits (vancomycin 2–400 mg/ml, tobramycin 0.5–20 mg/ml). Additionally, without the drains, higher concentrations of antibiotic would be expected locally for longer periods of time; however, drains are standard of care for patients with severe osteomyelitis. The exact values for antibiotic concentrations above the upper limits were not recorded for all patients. Therefore, the data do not give an accurate evaluation of local antibiotic concentrations in these cases. However, the data do indicate that these reported upper limits were present usually for only 1–2 days post-implantation. Thus, the use of antibiotic concentrations from eluent to indicate the diminishing local antibiotic concentrations over the 5-day period remains possible. In addition, the blood serum concentrations indicate that these high local concentrations post-implantation do not manifest into high systemic antibiotic concentrations. Moreover, additional laboratory data such as hepatic function, coagulation studies, and comorbidities would have been useful in controlling for any other variables that could confound the systemic antibiotic concentration measurements. However, the data were collected at the time of the initial study, and retrieval of the other information is not possible because of the deletion of the hospital records given some patient records eclipsed the 8-year maximum storage rule by the hospital system. Another limitation is that this study did not control for variability of the amount of antibiotic based on the volumetric size of the surgical bed. For example, larger surgical beds with the same number of pellets as smaller surgical beds will have smaller areas of antibiotic diffusion.
5 Conclusions
Based on our results and clinical experience, the antibiotic-loaded pure calcium sulfate hemihydrate demonstrates adequacy as a platform for the local delivery of antibiotics at therapeutic concentrations as well as a stable vehicle for incorporation of both vancomycin and tobramycin. In each of the 5 postoperative days evaluated, mean local concentrations of antibiotics exceeded values capable of inhibiting common pathogens. We observed no adverse reaction based on the presence of elevated serum concentrations of antibiotics and no occurrence of persistent wound drainage associated with the antibiotic-loaded pure calcium sulfate hemihydrate.
References
Olson ME, Garvin KL, Fey PD, Rupp ME. Adherence of Staphylococcus epidermidis to biomaterials is augmented by PIA. Clin Orthop Relat Res. 2006;451:21–4.
Barton AJ, Sagers RD, Pitt WG. Measurement of bacterial growth rates on polymers. J Biomed Mater Res. 1996;32:271–8.
MacKintosh EE, Patel JD, Marchant RE, Anderson JM. Effects of biomaterial surface chemistry on the adhesion and biofilm formation of Staphylococcus epidermidis in vitro. J Biomed Mater Res A. 2006;78:836–42.
Rohde H, Frankenberger S, Zähringer U, Mack D. Structure, function and contribution of polysaccharide intercellular adhesin (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur J Cell Biol. 2010;89:103–11.
Gogia JS, Meehan JP, Di Cesare PE, Jamali AA. Local antibiotic therapy in osteomyelitis. Semin Plast Surg. 2009;23(2):100–7.
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–45.
Fraimow HS. Systemic antimicrobial therapy in osteomyelitis. Semin Plast Surg. 2009;23(2):90–9.
Lazar V, Chifiriuc MC. Medical significance and new therapeutical strategies for biofilm associated infections. Roum Arch Microbiol Immunol. 2010;69(3):125–38.
Davis SC, Ricotti C, Cazzaniga A, Welsh E, et al. Microscopic and physiologic evidence for biofilmassociated wound colonization in vivo. Wound Rep Reg. 2008;16:23–9.
Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumonia biofilm resistance to ampicillin and ciproflacin. Antimicrob Agents Chemother. 2000;44(7):1818–24 (Level II).
Widgerow AD. Persistence of the chronic wound: implicating biofilm. Wound Heal South Afr. 2008;1(2):5–7.
Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg. 2002;10(1):53–60.
Le Ray AM, Gautier H, Laty MK, Daculsi G, Merle C, Jacqueline C, Hamel A, Caillon J. In vitro and in vivo bactericidal activities of vancomycin dispersed in porous biodegradable poly(ὲ-caprolactone) microparticles. Antimicrob Agents Chemother. 2005;49(7):3025–7.
Kent ME, Rapp RP, Smith KM. Antibiotic beads and osteomyelitis: here today, what’s coming tomorrow? Orthopedics. 2006;29(7):599.
Holtom PD, Patzakis MJ. Newer methods of antibiotic delivery for bone and joint infections. Instr Course Lect. 2003;52:745–9.
Maale GE, Pascoe HR, Piercy EA. A standardized approach for the treatment of infected total joint arthroplasties by the DFW sarcoma group osteomyelitis protocol; staged revisions at 2 weeks using antibiotic-cement-implant composites as spacers. J Jt Arthroplast. 1993;8(1):102.
Cierny G III, Mader JT, Pennick JJ. A clinical staging system for adult osteomyelitis. Clin Orthop Relat Res. 2003;414:7–24. https://doi.org/10.1097/01/blo.0000088564.81746.62(Medline).
Kanellakopoulou K, Giamarellos-Bourboulis EJ. Carrier systems for local delivery of antibiotics in bone infections. Drugs. 2000;59(6):1223–322.
Zalavras CG, Patzakis MJ, Holtom P. Local antibiotic therapy in the treatment of open fractures and osteomyelitis. Clin Orthop Relat Res. 2004;427:86–93.
Van de Belt H, Neut D, Schenck W, van Horn JR, van der Mei HC, Busscher HJ. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. A review. Acta Orthop Scand. 2001;72(6):557–71.
Masri BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone-cement: an in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplast. 1998;13:331–8.
Nelson CL. The current status of material used for depot delivery of drugs. Clin Orthop Relat Res. 2004;427:72–8 (Level II).
Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma. 2004;56:1247–52.
Meek RM, Masri BA, Dunlop D, Garbuz DS, Greidanus NV, McGraw R, Duncan CP. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Jt Surg Am. 2003;85:1888–922.
El-Husseiny M, Patel S, MacFarlane RJ, Haddad FS. Biodegradable antibiotic delivery systems. J Bone Jt Surg Br. 2011;93(2):151–7.
Webb ND, McCanless JD, Courtney HS, Bumgardner JD, Haggard WO. Daptomycin eluted from calcium sulfate appears effective against staphylococcus. Clin Orthop Relat Res. 2008;466:1383–7.
Liu SJ, Wen-Neng Ueng S, Lin SS, Chan EC. In vivo release of vancomycin from biodegradable beads. J Biomed Mater Res. 2002;63(6):807–13.
Kinnari TJ, Esteban J, Zamora N, et al. Effect of surface roughness and sterilization on bacterial adherence to ultra-high molecular weight polyethylene. Clin Microbiol Infect. 2010;16:1036–41.
Patrick BN, Rivey MP, Allington DR. Acute renal failure associated with vancomycin and tobramycin laden cement in total hip arthroplasty. Ann Pharmacother. 2006;40(11):2037–42.
Calhoun JH, Mader JT. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthop Relat Res. 1997;341:206–14.
Kanellakopoulou K, Panagopoulos P, Giannitsioti E, Tsaganos T, Carrer DP, Efstathopoulos N, Giamarellos-Bourboulis EJ. In vitro elution of daptomycin by a synthetic crystallic semihydrate form of calcium sulfate, Stimulan. Antimicrob Agents Chemother. 2009;53(7):3106–7.
Richelsoph KC, Webb ND, Haggard WO. Elution behavior of daptomycin loaded calcium sulfate pellets: a preliminary study. Clin Orthop Relat Res. 2007;461:68–73.
Kelly CM, et al. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001;382:42–50.
Grimsrud C, et al. The in vitro elution characteristics of antifungal-loaded PMMA bone cement and calcium sulfate bone substitute. Orthopedics. 2011;34(8):e378–e381381.
Shirtliff ME, Calhoun JH, Mader JT. Experimental osteomyelitis treatment with antibiotic impregnated hydroxyapatite. Clin Orthop Relat Res. 2002;401:239–47 (Level II).
Sanicola SM, Albert SF. The in vitro elution characteristics of vancomycin and tobramycin from calcium sulfate beads. J Foot Ankle Surg. 2005;44(2):121–4 (Level II).
Scharer BM, Sanicola SM. The in vitro elution characteristics of vancomycin from calcium phosphate-calcium sulfate beads. J Foot Ankle Surg. 2009;48(5):540–2.
Cai X, et al. The use of calcium sulfate impregnated with vancomycin in the treatment of open fractures of long bones: a preliminary study. Orthopedics. 2010;33(3):152–7.
Beuerlein MJ, Mckee MD. Calcium sulfates: what is the evidence?. J Orthop Trauma. 2010;24(Suppl 1):S46–51.
Cierny G III, DiPasquale D. Comparing OsteoSet and Stimulan as antibiotic-loaded, Calcium sulfate beads in the management of musculoskeletal infection. Proceedings of the 19th Meeting of the Musculoskeletal Infection Society, 2009 Aug 7–8; San Diego, California, USA. Abstract: 27. Avaialble from: https://www.msis-na.org/annual-meetings/2009-annual-meeting/2009-abstracts/2009-abstract-27/. Accessed 20 Apr 2020.
ThermoNicolet. Introduction to Fourier transform infrared spectrometry. Madison: ThermoNicolet; 2001.
Rice D, Mendez-Vigo L. Daptomycin in bone and joint infections: a review of literature. Arch Orthop Trauma Surg. 2009;129:1495–504.
Abbott. Architect c8000 operations manual. Abbott Park: Abbott Core Laboratory; 2005.
Dienstag J, Neu H. In vitro studies of tobramycin, an aminoglycoside antibiotic. Antimicrob Agents Chemother. 1972;1:41–5.
Jumah MTB, Vasoo S, Menon SR, De PP, Neely M, Teng CB. Pharmacokinetic/pharmacodynamic determinants of vancomycin efficacy in enterococcal bacteremia. Antimicrob Agents Chemother. 2018;62:e01602–e1617.
Prakash V, Lewis J II, Jorgensen J. Vancomycin MICs for methicillin-resistant Staphylococcus aureus isolates differ based upon the susceptibility test method used. Antimicrob Agents Chemother. 2008;2008(52):4528.
Camara M, Dieng A, Boye CS. Antibiotic susceptibility of streptococcus pyogenes isolated from respiratory tract infections in dakar, senegal. Microbiol Insights. 2013;6:71–5.
Gerhard EM. Débridement for orthopaedic infection. In: Wellington KH, Alex CM, Bryan D, editors. Let’s discuss surgical site infections, Chap. 5. Rosemont: American Academy of Orthopaedic Surgeons (AAOS); 2015.
Maale GE, Eager JJ. Local elution profiles of a highly purified calcium sulfate pellet at physiologic pH, loaded with vancomycin and tobramycin, in the treatment of infected total joints. In: the 75th Annual meeting of the Western Orthopaedic Association, Honolulu, HI, USA; 2011.
Acknowledgements
Pamela Jean Jensen MD. PamelaJensen@texashealth.org.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No source of funding was used to conduct this study.
Conflict of interest
Dr. Maale has received royalty rights with Biocomposites Ltd. until April 1, 2020 and royalty rights currently with Smith and Nephew on the legion hk system. All other authors have no conflict of interest to declare.
Ethics approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration (and its amendments) and the details of the Ethics Committee or institutional review board which approved the study. The study was approved by the Institutional Review Board at Medical City Dallas.
Informed consent
At the time of the study, no additional consents were obtained. All consent related to the study was incorporated into the operative consent form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Maale, G.E., Eager, J.J., Mohammadi, D.K. et al. Elution Profiles of Synthetic CaSO4 Hemihydrate Beads Loaded with Vancomycin and Tobramycin. Eur J Drug Metab Pharmacokinet 45, 547–555 (2020). https://doi.org/10.1007/s13318-020-00622-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00622-8