Abstract
Background and Objective
Lithium, which is used to treat bipolar disorder, has a narrow therapeutic blood concentration range and quickly reaches clinically toxic levels. We performed a population pharmacokinetic analysis with a lithium tubular reabsorption model including urinary pH and investigated the relationship between blood lithium concentration and tremor as a side effect.
Methods
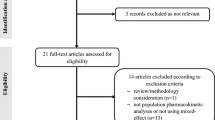
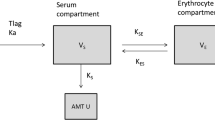
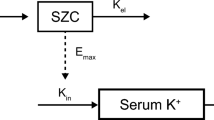
Routine clinical data, including 389 serum concentrations, were collected from 214 patients orally administered an adjusted amount of lithium carbonate. Pharmacokinetics were described using a one-compartment distribution model with first-order absorption and elimination. The fractions of the MID (Li+ + LiCO3−) and ION (2Li+ + CO32−) forms were calculated using the Henderson–Hasselbalch equation, and the influences of these fractions on clearance (CL) were evaluated. The rate of tremor development was analyzed using a logit model.
Results
Oral apparent CL (CL/F) was explained by nonrenal CL and renal CL, and renal CL was varied by the fractions of lithium forms influenced by urinary pH. The contribution of MID to CL was slightly larger than that of ION. The rate of tremor development was estimated to be more than 30% when the trough lithium concentration was greater than 1.26 mEq L−1.
Conclusion
Renal function and urinary pH are important indices in lithium treatment, so the serum concentration of lithium may be predicted based on the renal function and urinary pH.





Similar content being viewed by others
References
Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–52.
Schou M. Lithium in psychiatric therapy and prophylaxis. J Psychiatr Res. 1968;6:67–95.
Grandjean EM, Aubry J-M. Lithium: updated human knowledge using an evidence-based approach. Part I: clinical efficacy in bipolar disorder. CNS Drugs. 2009;23:225–40.
Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381:1672–82.
Smith LA, Cornelius V, Warnock A, Tacchi MJ, Taylor D. Pharmacological interventions for acute bipolar mania: a systematic review of randomized placebo-controlled trials. Bipolar Disord. 2007;9:551–60.
Yildiz A, Vieta E, Leucht S, Baldessarini RJ. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36:375–89.
Schou M. Pharmacology and toxicology of lithium. Annu Rev Pharmacol Toxicol. 1976;16:231–43.
Thomsen K, Olesen OV. Precipitating factors and renal mechanisms in lithium intoxication. Gen Pharmacol. 1978;9:85–9.
Thomsen K. Renal handling of lithium at non-toxic and toxic serum lithium levels. A review. Dan Med Bull. 1978;25:106–15.
Kanba S, Kato T, Terao T, Yamada K. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285–300.
Schou M, Amdisen A, Trap-Jensen J. Lithium poisoning. Am J Psychiatr. 1968;125:520–7.
Prien RF, Caffey EM, Klett CJ. Relationship between serum lithium level and clinical response in acute mania treated with lithium. Br J Psychiatry. 1972;120:409–14.
Gelenberg A, et al. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med. 1989;321:1489–93.
McKnight RF, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721–8.
Watanabe S. Side effects of antimanic drugs. Jpn J Neuropsychopharmacol. 1989;11:49–58.
Ebid AIM, Abd-allah DAT, Elhabiby MMM. Pharmacokinetics of lithium in Egyptian bipolar patients: dosage adjustment approach. Pharmacol Pharm. 2014;5:425–32.
Henderson L. Concerning the relationship between the strength of acids and their capacity to preserve neutrality. Am J Physiol. 1908;21:173–9.
Hasselbalch K. Die Berechnung der Wasserstoffzahl des Blutes aus der freien und gebundenen Kohlensäure desselben, und die Sauerstoffbindung des Blutes als Funktion der Wasserstoffzahl. Biochem Z. 1917;78:112–44.
Anderson BJ, Meakin GH. Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth. 2002;12:205–19.
Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–52.
Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36.
Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Mould DR, et al. Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther. 2002;71:334–48.
Matthews I, Kirkpatrick C, Holford N. Quantitative justification for target concentration intervention—parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol. 2004;58:8–19.
Parke J, Holford NHG, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999;59:19–29.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Thornhill DP. Pharmacokinetics of ordinary and sustained-release lithium carbonate in manic patients after acute dosage. Eur J Clin Pharmacol. 1978;14:267–71.
Turck D, Heinzel G, Luik G. Steady-state pharmacokinetics of lithium in healthy volunteers receiving concomitant meloxicam. Br J Clin Pharmacol. 2000;50:197–204.
Nielsen-Kudsk F, Amedisen A. Analysis of the pharmacokinetics of lithium in man. Eur J Clin Pharmacol. 1979;16:271–7.
Yukawa E, Nomiyama N, Higuchi S, Aoyama T. Lithium population pharmacokinetics from routine clinical data: role of patient characteristics for estimating dosing regimens. Ther Drug Monit. 1993;15:75–82.
Wing YK, Chan E, Chan K, Lee S, Shek CC. Lithium pharmacokinetics in Chinese manic-depressive patients. J Clin Psychopharmacol. 1997;17:179–84.
Findling RL, et al. First-dose pharmacokinetics of lithium carbonate in children and adolescents. J Clin Psychopharmacol. 2010;30:404–10.
Vesterqaard P, Poulstrup I, Schou M. Prospective studies on a lithium cohort. 3. Tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatr Scand. 1988;78:434–41.
Feng Y, et al. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol. 2014;78:106–17.
Author information
Authors and Affiliations
Contributions
DY and YT contributed to the acquisition of data, analyzed and interpreted data, participated in the study design, and drafted the manuscript. CO analyzed and interpreted data and revised the manuscript. HK, HT, and HK contributed to the conception and design of the study and the interpretation of data. MS, SK, and HK contributed to serum concentration measurements and patient data sampling. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Funding
This study was supported by a grant from SENSHIN Medical Research Foundation and Takeda Science Foundation.
Conflict of interest
The authors (Daichi Yamaguchi, Yasuhiro Tsuji, Miki Sonoda, Kenji Shin, Hiroko Kito, Chika Ogami, Hidefumi Kasai, Hideto To, and Hidetoshi Kamimura) declare no conflict of interest. All authors have completed the Unified Competing Interest form and declare that there was no support from any organization for the submitted work, no financial relationships with any organizations that may have an interest in the submitted work in the previous 3 years, and no other relationships or activities that may have influenced the submitted work.
Ethics approval
The present study was performed in accordance with the Helsinki Declaration after approval by the ethical review board of the University of Toyama (approval number: clinical 26-39). It was then approved by Yahata Kousei Hospital, Iizuka Hospital, and Fukuma Hospital.
Informed consent
Written consent was obtained from all patients, and patient privacy and personal information were respected.
Rights and permissions
About this article
Cite this article
Yamaguchi, D., Tsuji, Y., Sonoda, M. et al. Population Pharmacokinetics and Exposure–Response of Lithium Carbonate in Patients Based on Tubular Reabsorption Mechanisms. Eur J Drug Metab Pharmacokinet 44, 329–338 (2019). https://doi.org/10.1007/s13318-018-0536-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0536-0