Abstract
Background
Requirements for predicting human pharmacokinetics in drug discovery are increasing. Developing different methods of human pharmacokinetic prediction will facilitate lead optimization, candidate nomination, and dosing regimens before clinical trials at various early drug discovery stages.
Objectives
To develop and validate generic methods of human pharmacokinetic prediction to meet the requirements in early drug discovery.
Methods
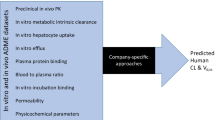
The physiologically based pharmacokinetic (PBPK) model implemented in Gastroplus™ was used for human pharmacokinetic predictions. The absorption, distribution, metabolism, and excretion properties of drugs in humans predicted from molecular structure and extrapolated from tested preclinical data were used as inputs in the PBPK model. The approaches were validated by comparison of the predicted pharmacokinetic parameters with actual pharmacokinetic parameters of 15 marketed small-molecule compounds approved by the US Food and Drug Administration. Based on the validation and reported approaches, we proposed a strategy for human pharmacokinetic prediction at different drug discovery stages.
Results
Obvious underestimation of exposure (< 1/3 of actual exposure) was not observed using in silico prediction as inputs, which may reduce the probability of missing the potential compounds with predicted false low exposure. The simulated human pharmacokinetic results using tested data as inputs were superior to those obtained via in silico prediction. Both methods similarly predicted the multiphasic shape of pharmacokinetic profiles.
Conclusion
These generic PBPK approaches of full in silico prediction or perdition using a combination of tested in vivo and in vitro data were validated and proved useful for human pharmacokinetic predictions.




Similar content being viewed by others
References
Dedrick RL. Animal scale-up. J Pharmacokinet Biopharm. 1973;1:435–61.
Wajima T, Yano Y, Fukumura K, et al. Prediction of human pharmacokinetic profile in animal scale up based on normalizing time course profiles. J Pharm Sci. 2004;93:1890–900.
Jones HM, Gardner IB, Collard WT, et al. Simulation of human intravenous and oral pharmacokinetics of 21 diverse compounds using physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2011;50:331–47.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45:1013–34.
Hosea NA, Collard WT, Cole S, et al. Prediction of human pharmacokinetics from preclinical information: comparative accuracy of quantitative prediction approaches. J Clin Pharmacol. 2009;49:513–33.
Hao K, Qi Q, Wan P, et al. Prediction of human pharmacokinetics from preclinical information of rhein, an antidiabetic nephropathy drug, using a physiologically based pharmacokinetic model. Basic Clin Pharmacol Toxicol. 2014;114:160–7.
Li R, Ghosh A, Maurer TS, et al. Physiologically based pharmacokinetic prediction of telmisartan in human. Drug Metab Dispos. 2014;42:1646–55.
Liu F, Zhuang X, Yang C, et al. Characterization of preclinical in vitro and in vivo ADME properties and prediction of human PK using a physiologically based pharmacokinetic model for YQA-14, a new dopamine D3 receptor antagonist candidate for treatment of drug addiction. Biopharm Drug Dispos. 2014;35:296–307.
Wang B, Liu Z, Li D, et al. Application of physiologically based pharmacokinetic modeling in the prediction of pharmacokinetics of bicyclol controlled-release formulation in human. Eur J Pharm Sci. 2015;77:265–72.
De Buck SS, Sinha VK, Fenu LA, et al. Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos. 2007;35:1766–800.
Jones HM, Parrott N, Jorga K, et al. A novel strategy for physiologically based predictions of human pharmacokinetics. Clin Pharmacokinet. 2006;45:511–42.
Parrott N, Paquereau N, Coassolo P, et al. An evaluation of the utility of physiologically based models of pharmacokinetics in early drug discovery. J Pharm Sci. 2005;94:2327–43.
Jones HM, Gardner IB, Watson KJ. Modelling and PBPK simulation in drug discovery. AAPS J. 2009;11:155–66.
Theil FP, Guentert TW, Haddad S, et al. Utility of physiologically based pharmacokinetic models to drug development and rational drug discovery candidate selection. Toxicol Lett. 2003;138:29–49.
Zou P, Yu Y, Zheng N, et al. Applications of human pharmacokinetic prediction in first-in-human dose estimation. AAPS J. 2012;14:262–81.
Brightman FA, Leahy DE, Searle GE, et al. Application of a generic physiologically based pharmacokinetic model to the estimation of xenobiotic levels in human plasma. Drug Metab Dispos. 2006;34:94–101.
Hu T-M, Chiu S-J. Prediction of human drug clearance using a single-species, fixed-exponent allometric approach. J Med Sci. 2009;29:9.
Sohlenius-Sternbeck AK, Afzelius L, Prusis P, et al. Evaluation of the human prediction of clearance from hepatocyte and microsome intrinsic clearance for 52 drug compounds. Xenobiotica. 2010;40:637–49.
Zanelli U, Caradonna NP, Hallifax D, et al. Comparison of cryopreserved HepaRG cells with cryopreserved human hepatocytes for prediction of clearance for 26 drugs. Drug Metab Dispos. 2012;40:104–10.
Berellini G, Waters NJ, Lombardo F. In silico prediction of total human plasma clearance. J Chem Inf Model. 2012;52:2069–78.
Yamagata T, Zanelli U, Gallemann D, et al. Comparison of methods for the prediction of human clearance from hepatocyte intrinsic clearance for a set of reference compounds and an external evaluation set. Xenobiotica. 2017;47:741–51.
Beaumont K, Gardner I, Chapman K, et al. Toward an integrated human clearance prediction strategy that minimizes animal use. J Pharm Sci. 2011;100:4518–35.
Mahmood I. Application of fixed exponent 0.75 to the prediction of human drug clearance: an inaccurate and misleading concept. Drug Metabol Drug Interact. 2009;24:57–81.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94:1259–76.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95:1238–57.
Mahmood I. Interspecies scaling of biliary excreted drugs: prediction of human clearance and volume of distribution. Drug Metabol Drug Interact. 2012;27:157–64.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/201292Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205755Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203756Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206192Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202570Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202570Orig1s000ClinPharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/202806Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021986s000_Sprycel__PharmR.pdf. Accessed 26 Jan 2018.
Tsume Y, Takeuchi S, Matsui K, et al. In vitro dissolution methodology, mini-gastrointestinal simulator (mGIS), predicts better in vivo dissolution of a weak base drug, dasatinib. Eur J Pharm Sci. 2015;76:203–12.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-399_IRESSA_Pharmr_P1.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-335_Gleevec_pharmr_P1.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/206947Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.pmda.go.jp/files/000210264.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205832Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021938_S000_Sutent_PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021923_s000_Nexavar_PharmR.pdf. Accessed 26 Jan 2018.
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203214Orig1s000PharmR.pdf. Accessed 26 Jan 2018.
Darwich AS, Margolskee A, Pepin X, et al. IMI—Oral biopharmaceutics tools project—Evaluation of bottom-up PBPK prediction success part 3: identifying gaps in system parameters by analysing in silico performance across different compound classes. Eur J Pharm Sci. 2017;96:626–42.
Gobeau N, Stringer R, De Buck S, et al. Evaluation of the GastroPlus™ Advanced Compartmental and Transit (ACAT) model in early discovery. Pharm Res. 2016;33:2126–39.
Acknowledgements
We thank Simulations Plus, Inc for authorizing the use of ADMET PredictorTM program for this study. We thank Professor Guo-zhu Han (Dalian Medical University, China) for his review and helpful comments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest directly relevant to the content of this study to declare.
Funding
No funding has been used for this study.
Rights and permissions
About this article
Cite this article
Ren, HC., Sai, Y. & Chen, T. Evaluation of Generic Methods to Predict Human Pharmacokinetics Using Physiologically Based Pharmacokinetic Model for Early Drug Discovery of Tyrosine Kinase Inhibitors. Eur J Drug Metab Pharmacokinet 44, 121–132 (2019). https://doi.org/10.1007/s13318-018-0496-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0496-4