Abstract
Background and Objective
Nefopam is a non-opioid, non-steroidal, central analgesic thought to act via multiple mechanisms including potent inhibition of serotonin–norepinephrine reuptake and modulation of voltage-sensitive calcium and sodium channels. There has been a resurgence in its use for postoperative pain and neuropathic pain. Dosing route-dependent metabolism and clinical effects have been described following intravenous and oral nefopam. N-desmethylnefopam and nefopam N-oxide are metabolites of clinical interest. We sought to develop a joint pharmacokinetic model to simultaneously describe the plasma and urinary pharmacokinetics of nefopam and the two metabolites following an oral pharmacological dose of [14C]-nefopam to healthy volunteers, and to estimate inter-individual variability in their pharmacokinetics.
Methods
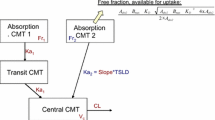
Pharmacokinetic data for the parent and metabolites were analyzed simultaneously using NONMEM® (nonlinear mixed-effect modeling) v7.3. The modeling process evaluated, in part, one- and two-compartment linear pharmacokinetic models for nefopam and a single compartment for each of the two metabolites. Pathways for presystemic metabolism of both metabolites were explored.
Results
The final structural model simultaneously described the plasma and urinary pharmacokinetics of nefopam and the two metabolites. It consists of a central compartment for nefopam and for each of the two metabolites, as well as a peripheral compartment for the parent, and the associated urine compartments. The rapid formation and appearance of the N-oxide in plasma, characterized by concentrations that peak earlier than the parent, could be described by presystemic formation in the gastrointestinal tract.
Conclusions
A descriptive, robust and predictive parent–metabolite model has been developed using a population mixed-effects approach to characterize the pharmacokinetics of nefopam and its metabolites simultaneously in healthy subjects following oral administration of nefopam. The model may be used for dose selection, analysis of sparse data, identification of intrinsic and extrinsic factors, and to model the clinical effects of each analyte.









Similar content being viewed by others
References
Girard P, Chauvin M, Verleye M. Nefopam analgesia and its role in multimodal analgesia: a review of preclinical and clinical studies. Clin Exp Pharmacol Physiol. 2016;43(1):3–12.
Aymard G, Warot D, Démolis P, Giudicelli JF, Lechat P, Le Guern ME, Alquier C, Diquet B. Comparative pharmacokinetics and pharmacodynamics of intravenous and oral nefopam in healthy subjects. Pharmacol Toxicol. 2003;92:279–86.
Sanga M, Banach J, Ledvina A, Modi NB, Mittur A. Pharmacokinetics, metabolism, and excretion of nefopam, a dual reuptake inhibitor in healthy male volunteers. Xenobiotica. 2016;46(11):1001–16.
Chawla J, Le Guern ME, Alquier C, Kalhorn TF, Levy RH. Effect of route of administration on the pharmacokinetic behavior of enantiomers of nefopam and desmethylnefopam. Ther Drug Monit. 2003;25:203–10.
Mimoz O, Chauvet S, Grégoire N, Marchand S, Le Guern ME, Saleh A, Couet W, Debaene B, Levy RH. Nefopam pharmacokinetics in patients with end-stage renal disease. Anest Analg. 2010;111:1146–53.
Djerada Z, Fournet-Fayard A, Gozalo C, Lelarge C, Lamiable D, Millart H, Malinovsky JM. Population pharmacokinetics of nefopam in elderly, with or without renal impairment, and its link to treatment response. Br J Clin Pharmacol. 2014;77:1027–38.
Kirchherr HJ, Christ W. Nefopam and N-desmethyl-nefopam: activity in the hot plate and writhing test. Naunyn Schmiedebergs Arch Pharmacol. 1984;325(suppl.):R72.
Bhatt AM, Pleuvry BJ, Maddison SE. Respiratory and metabolic effects of oral nefopam in human volunteers. Br J Clin Pharmacol. 1981;11(2):209–11.
Saccomani MP, Audoly S, Bellu G, D’Angiò L. Examples of testing global identifiability of biological and biomedical models with the DAISY software. Comput Biol Med. 2010;40(4):402–7.
Savic RM, Karlsson MO. Importance of shrinkage in empirical bayes estimates for diagnostics: problems and solutions. AAPS J. 2009;11(3):558–69.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;2:e38.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–51.
Dosne AG, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43(6):583–96.
Podranski T, Bouillon TW, Riva T, Kurz AM, Oehmke MJ. Compartmental pharmacokinetics of nefopam during mild hypothermia. Br J Anaesth. 2012;108(5):784–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by Impax Specialty Pharma, a Division of Impax Laboratories, Inc.
Conflict of interest
Aravind Mittur is an employee of Impax Laboratories, Inc. and holds Impax stock.
Ethics approval
The study was conducted in full compliance with the 1964 Helsinki Declaration (and its amendments) and the International Conference on Harmonization Good Clinical Practices guidelines. The study protocol, consent documents, consent procedures, and subject recruitment procedures were approved by the institutional review board.
Informed consent
All study participants provided written informed consent before participation in the study.
Rights and permissions
About this article
Cite this article
Mittur, A. A Simultaneous Mixed-Effects Pharmacokinetic Model for Nefopam, N-desmethylnefopam, and Nefopam N-Oxide in Human Plasma and Urine. Eur J Drug Metab Pharmacokinet 43, 391–404 (2018). https://doi.org/10.1007/s13318-017-0457-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0457-3