Abstract
Background and Objectives
Crassicauline A, a C19 diterpenoid alkaloid in Aconitum herbs, is an analgesic drug clinically used in China. The in vivo metabolism of crassicauline A is poorly understood, while potential bioactivation is anticipated via hydroxylation metabolism. This work, therefore, aimed to investigate the in vivo hydroxylation metabolism of crassicauline A in rats.
Methods
Using a de novo developed and validated UPLC–MS/MS method, excretion studies in rats were carried out to investigate the recoveries of crassicauline A and its hydroxylated metabolites in urine and feces. Mass fragmentation analysis was used to identify the detected hydroxylated metabolites. In vitro metabolism assay in liver S9 fraction was employed to preliminarily investigate the inter-species difference of hydroxylation metabolism between rats and human.
Results
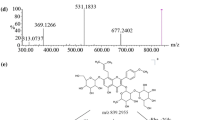
At a toxic dose of 100 µg/kg, less than 10% and 5% of the administrated dose of crassicauline A were recovered in the urine and feces after single intravenous and oral administration, respectively. Trace of yunaconitine, a possible 3-hydroxylated metabolite of crassicauline A, was detected in urine samples, but not considered to be derived from the in vivo metabolism, because the recovered yunaconitine and crassicauline A was equivalent to their occurrences in the test article. Another hydroxylated metabolite was detected with much higher levels than yunaconitine. Based on chromatographic behaviors and fragmentation analysis, the hydroxylation site of this metabolite was tentatively identified at C-15 on the skeleton, which might have produced a toxic alkaloid known as deoxyjesaconitine. The in vivo observations were consistent with the preliminary in vitro results in liver S9 fraction, in which an inter-species difference was highlighted that rats demonstrated more hydroxylation than human did.
Conclusions
This work disclosed that crassicauline A is elimilated in rats predominantly by metabolism under toxic dosage and the hydroxylation probably at C-15 might be a potential bioactivation pathway in both rats and human.






Similar content being viewed by others
References
Fengpeng W, Qicheng F. Alkaloids from roots of Aconitum crassicaule. Planta Med. 1981;42(8):375–9. doi:10.1055/s-2007-971658.
Tang XC. Anti-inflammatory and analgesic new drug: bulleyaconitine A. Xin Yao Yu Lin Chuang. 1986;5(2):120–1.
Tang XC, Liu XJ, Lu WH, Wang MD, Li AL. Studies on the analgesic action and physical dependence of bulleyaconitine A. Yao Xue Xue Bao. 1986;21(12):886–91.
Wang CF, Gerner P, Wang SY, Wang GK. Bulleyaconitine A isolated from aconitum plant displays long-acting local anesthetic properties in vitro and in vivo. Anesthesiology. 2007;107(1):82–90. doi:10.1097/01.anes.0000267502.18605.ad.
Weng W, Xu H, Huang J, Wang G, Shen T, Zhang J. Determination of bulleyaconitine A in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;816(1–2):315–20. doi:10.1016/j.jchromb.2004.11.051.
Weng WY, Xu HN, Huang JM, Wang GQ, Shen T, Zhang JF. A pharmacokinetic study of intramuscular administration of bulleyaconitine A in healthy volunteers. Biol Pharm Bull. 2005;28(4):747–9.
Wang Q, Tan B, Gong Y, Ji G, Zhang Y, Yang P, et al. Determination of bulleyaconitine A in plasma by a sensitive LC–MS/MS method and its application to an oral pharmacokinetic study in rats. J Pharm Biomed Anal. 2012;71:202–6. doi:10.1016/j.jpba.2012.08.016.
Wang FP, Chen QH, Liu XY. Diterpenoid alkaloids. Nat Prod Rep. 2010;27(4):529–70. doi:10.1039/b916679c.
Hikino H, Konno C, Takata H, Yamada Y, Yamada C, Ohizumi Y, et al. Antiinflammatory principles of Aconitum roots. J Pharmacobiodyn. 1980;3(10):514–25.
Honerjager P, Meissner A. The positive inotropic effect of aconitine. Naunyn Schmiedebergs Arch Pharmacol. 1983;322(1):49–58.
Wang FP, Liang XT. The alkaloids: chemistry and pharmacology. New York: Academic Press; 1992.
Chan TY. Aconite poisoning. Clin Toxicol. 2009;47(4):279–85. doi:10.1080/15563650902904407.
Fu M, Wu M, Qiao Y, Wang Z. Toxicological mechanisms of Aconitum alkaloids. Pharmazie. 2006;61(9):735–41.
Zhang M, Peng CS, Li XB. In vivo and in vitro metabolites from the main diester and monoester diterpenoid alkaloids in a traditional chinese herb, the aconitum species. Evid Based Complement Alternat Med. 2015;2015:252434. doi:10.1155/2015/252434.
Lan K, Xie G, Jia W. Towards polypharmacokinetics: pharmacokinetics of multicomponent drugs and herbal medicines using a metabolomics approach. Evid Based Complement Alternat Med. 2013;2013:819147. doi:10.1155/2013/819147.
Wang JL, Shen XL, Chen QH, Qi G, Wang W, Wang FP. Structure-analgesic activity relationship studies on the C(18)- and C(19)-diterpenoid alkaloids. Chem Pharm Bull (Tokyo). 2009;57(8):801–7.
Wang FP, Liang XT. The alkaloids: chemistry and biology. New York: Academic Press; 2001.
Bi Y, Zhuang X, Zhu H, Song F, Liu Z, Liu S. Studies on metabolites and metabolic pathways of bulleyaconitine A in rat liver microsomes using LC–MS(n) combined with specific inhibitors. Biomed Chromatogr. 2015;29(7):1027–34. doi:10.1002/bmc.3388.
Food and Drug Administration. Guidance for industry: bioanalytical method validation (DRAFT GUIDANCE, Revision 1). 2013. https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm368107.pdf. Retrieved on Feb 25, 2017.
Hill JR. In vitro drug metabolism using liver microsomes. Curr Protoc Pharmacol. 2004;Unit 7.8.
Wang Z, Wen J, He Y. Simultaneous determination of three aconitum alkaloids in urine by LC–MS–MS. J Pharm Biomed Anal. 2007;45(1):145–8. doi:10.1016/j.jpba.2007.04.016.
Ka-Wing Chung K, Pak-Lam Chen S, Ng SW, Wing-Lai Mak T, Sze-Yin Leung K. Measurement of yunaconitine and crassicauline A in small-volume blood serum samples by LC–MS/MS: tracing of aconite poisoning in clinical diagnosis. Talanta. 2012;97:491–8. doi:10.1016/j.talanta.2012.05.004.
Acknowledgements
The authors thank Prof. Wang F. P. and Associate Prof. Chen D. L. from West China School of Pharmacy, Sichuan University for gifting 3-deoxyaconitine. This work was funded by the National Natural Science Foundation of China (No. 81302844).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by the National Natural Science Foundation of China (No. 81302844).
Conflict of interest
Xue-Jing Li is an employee Chengdu Health-Balance Pharmaceutical and Biomedical Tech. Co. Ltd. Kui Yang is an employee of Chengdu BaiKang Institute of Pharmacology and Toxicology. The authors report no additional conflicts of interest.
Ethical approval
The animal experiments were conducted in accordance with Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Ethical Committee of West China School of Pharmacy, Sichuan University. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Fan, X., Yin, SS., Li, XJ. et al. Hydroxylation Metabolisms of Crassicauline A in Rats Under Toxic Dose. Eur J Drug Metab Pharmacokinet 42, 857–869 (2017). https://doi.org/10.1007/s13318-017-0408-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0408-z