Abstract
Background and Objectives
Myelosuppression is a dose-limiting toxicity of 5-fluorouracil (5-FU). Predicting the inter- and intra-patient variability in pharmacokinetics and toxicities of 5-FU may contribute to the individualized medicine. This study aimed to establish a population pharmacokinetic–pharmacodynamic model that could evaluate the inter- and intra-individual variability in the plasma 5-FU concentration, 5-FU-induced body weight loss and myelosuppression in rats.
Method
Plasma 5-FU concentrations, body weight loss, and blood cell counts in rats following the intravenous administration of various doses of 5-FU for 4 days were used to develop the population pharmacokinetic–pharmacodynamic model.
Results
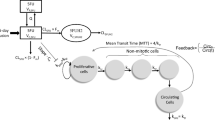
The population pharmacokinetic model consisting of a two-compartment model with Michaelis–Menten elimination kinetics successfully characterized the individual and population predictions of the plasma concentration of 5-FU and provided credible parameter estimates. The estimates of inter-individual variability in maximal rate of saturable metabolism and residual variability were 8.1 and 22.0%, respectively. The population pharmacokinetic–pharmacodynamic model adequately described the individual complete time-course of alterations in body weight loss, erythrocyte, leukocyte, and lymphocyte counts in rats treated with various doses of 5-FU. The inter-individual variability of the drug effects in the pharmacodynamic model for body weight loss was 82.6%, which was relatively high. The results of the present study suggest that not only individual fluctuations in the 5-FU concentration but also the cell sensitivity would affect the onset and degree of 5-FU-induced toxicity.
Conclusion
This population pharmacokinetic–pharmacodynamic model could evaluate the inter- and intra-individual variability in drug-induced toxicity and guide the assessments of novel anticancer agents in drug development.




Similar content being viewed by others
References
Kochi M, Akiyama Y, Aoki T, et al. FOLFIRI plus bevacizumab as a first-line treatment for Japanese patients with metastatic colorectal cancer: a JACCRO CC-03 multicenter phase II study. Cancer Chemother Pharmacol. 2013;72:1097–102.
Saif MW, Choma A, Salamone SJ, et al. Pharmacokinetically guided dose adjustment of 5-fluorouracil: a rational approach to improving therapeutic outcomes. J Natl Cancer Inst. 2009;101:1543–52.
Sakaeda T, Yamamori M, Kuwahara A, et al. Pharmacokinetics and pharmacogenomics in esophageal cancer chemoradiotherapy. Adv Drug Deliv Rev. 2009;61:388–401.
Ishikura S, Nihei K, Ohtsu A, et al. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–702.
Kumekawa Y, Kaneko K, Ito H, et al. Late toxicity in complete response cases after definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Gastroenterol. 2006;41:425–32.
Tahara M, Ohtsu A, Hironaka S, et al. Clinical impact of criteria for complete response (CR) of primary site to treatment of esophageal cancer. Jpn J Clin Oncol. 2005;35:316–23.
Tamura T, Kuwahara A, Kadoyama K, et al. Effects of bolus injection of 5-fluorouracil on steady-state plasma concentrations of 5-fluorouracil in Japanese patients with advanced colorectal cancer. Int J Med Sci. 2011;8:406–12.
Capitain O, Asevoaia A, Boisdron-Celle M, et al. Individual fluorouracil dose adjustment in FOLFOX base on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin Colorectal Cancer. 2012;11:263–7.
Gamelin E, Delva R, Jacob J, et al. Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2099–105.
Stec R, Bodnar L, Smoter M, et al. Mitomycin C and high-dose 5-fluorouracil with folinic acid as a therapeutic option for heavily pretreated patients with metastatic colorectal cancer: prospective phase II trial. Oncologist. 2014;19:356–7.
Kobuchi S, Ito Y, Hayakawa T, et al. Semi-physiological pharmacokinetic–pharmacodynamic modeling and simulation of 5-fluorouracil for the whole time course of alterations in leukocyte, neutrophil and lymphocyte counts in rats. Xenobiotica. 2014;44:804–18.
Kobuchi S, Ito Y, Hayakawa T, et al. Pharmacokinetic–pharmacodynamic (PK-PD) modeling and simulation of 5-fluorouracil for erythropenia in rats. J Pharmacol Toxicol Methods. 2014;70:134–44.
Kobuchi S, Ito Y, Hayakawa T, et al. Semi-physiological pharmacokinetic–pharmacodynamic (PK-PD) modeling and simulation of 5-fluorouracil for thrombocytopenia in rats. Xenobiotica. 2015;45:19–28.
Friberg LE, Freijs A, Sandström M, et al. Semiphysiological model for the time course of leukocytes after varying schedules of 5-fluorouracil in rats. J Pharmacol Exp Ther. 2000;295:734–40.
Kobuchi S, Ito Y, Okada K, et al. Pre-therapeutic assessment of plasma dihydrouracil/uracil ratio for predicting the pharmacokinetic parameters of 5-fluorouracil and tumor growth in a rat model of colorectal cancer. Biol Pharm Bull. 2013;36:907–16.
Kobuchi S, Ito Y, Okada K, et al. Pharmacokinetic/pharmacodynamic modeling of 5-fluorouracil by using a biomarker to predict tumor growth in a rat model of colorectal cancer. J Pharm Sci. 2013;102:2056–67.
Kobuchi S, Kuwano S, Imoto K, et al. A predictive biomarker for altered 5-fluorouracil pharmacokinetics following repeated administration in a rat model of colorectal cancer. Biopharm Drug Dispos. 2013;34:365–76.
Buchel B, Rhyn P, Schurch S, et al. LC-MS/MS method for simultaneous analysis of uracil, 5,6-dihydrouracil, 5-fluorouracil and 5-fluoro-5,6-dihydrouracil in human plasma for therapeutic drug monitoring and toxicity prediction in cancer patients. Biomed Chromatogr. 2013;27:7–16.
Mould DR, Upton RN. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacomet Syst Pharmacol. 2013;17:e38.
Chen J, Lu Q, Balthasar JP. Mathematical modeling of topotecan pharmacokinetics and toxicodynamics in mice. J Pharmacokinet Pharmacodyn. 2007;34:829–47.
Gao W, Jusko WJ. Modeling disease progression and rosiglitazone intervention in type 2 diabetic Goto-Kakizaki rats. J Pharmacol Exp Ther. 2012;341:617–25.
Friberg LE, Henningsson A, Maas H, et al. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol. 2002;20:4713–21.
Fuse E, Takai K, Okuno K, et al. Hepatic extraction ratio of 5-fluorouracil in rats. Dose dependence and effect of uracil and interleukin-2. Biochem Pharmacol. 1996;52:561–8.
Jarugula VR, Lam SS, Boudinot FD. Nonlinear pharmacokinetics of 5-fluorouracil in rats. J Pharm Sci. 1997;86:756–8.
van Kuilenburg AB, Maring JG. Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic drug monitoring in cancer patients. Pharmacogenomics. 2013;14:799–811.
Naguib FN, el Kouni MH, Cha S. Enzymes of uracil catabolism in normal and neoplastic human tissues. Cancer Res. 1985;45:5405–12.
Pinedo HM, Peters GF. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988;6:1653–64.
Kobuchi S, Ito Y, Nakano Y, et al. Population pharmacokinetic modelling and simulation of 5-fluorouracil incorporating a circadian rhythm in rats. Xenobiotica. 2016;46:597–604.
Baker SD, Verweij J, Rowinsky EK, et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J Natl Cancer Inst. 2002;94:1883–8.
Fety R, Rolland F, Barberi-Heyob M, et al. Clinical impact of pharmacokinetically-guided dose adaptation of 5-fluorouracil: results from a multicentric randomized trial in patients with locally advanced head and neck carcinomas. Clin Cancer Res. 1998;4:2039–45.
Undevia SD, Gomez-Abuin G, Ratain MJ. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer. 2005;5:447–58.
Jiang H, Lu J, Ji J. Circadian rhythm of dihydrouracil/uracil ratios in biological fluids: a potential biomarker for dihydropyrimidine dehydrogenase levels. Br J Pharmacol. 2004;141:616–23.
Ait-Oudhia S, Scherrmann JM, Krzyzanski W. Simultaneous pharmacokinetics/pharmacodynamics modeling of recombinant human erythropoietin upon multiple intravenous dosing in rats. J Pharmacol Exp Ther. 2010;334:897–910.
Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35:1672–8.
Woo S, Krzyzanski W, Jusko WJ. Target-mediated pharmacokinetic and pharmacodynamic model of recombinant human erythropoietin (rHuEPO). J Pharmacokinet Pharmacodyn. 2007;34:849–68.
Bender BC, Schaedeli-Stark F, Koch R, et al. A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol. 2012;70:591–601.
Chalret du Rieu Q, Fouliard S, Jacquet-Bescond A, et al. Application of hematological toxicity modeling in clinical development of abexinostat (S-78454, PCI-24781), a new histone deacetylase inhibitor. Pharm Res. 2013;30:2640–53.
Quartino AL, Friberg LE, Karlsson MO. A simultaneous analysis of the time-course of leukocytes and neutrophils following docetaxel administration using a semi-mechanistic myelosuppression model. Invest New Drugs. 2012;30:833–45.
Segura C, Bandrés E, Trocóniz IF, et al. Hematological response of topotecan in tumor-bearing rats: modeling of the time course of different cellular populations. Pharm Res. 2004;21:567–73.
Soto E, Staab A, Tillmann C, et al. Semi-mechanistic population pharmacokinetic/pharmacodynamic model for neutropenia following therapy with the Plk-1 inhibitor BI 2536 and its application in clinical development. Cancer Chemother Pharmacol. 2010;66:785–95.
Zandvliet AS, Schellens JH, Dittrich C, et al. Population pharmacokinetic and pharmacodynamic analysis to support treatment optimization of combination chemotherapy with indisulam and carboplatin. Br J Clin Pharmacol. 2008;66:485–97.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research (C) (No. 24590223) and a Grant-in-Aid for Young Scientists (B) (No. 15K18937) from MEXT (Ministry of Education, Culture, Sports, Science and Technology) of Japan.
Conflict of interest
SK, YI and TS have no conflicts of interests to declare.
Ethical approval
The experimental design was approved by an institutional review board prior to performing the research and all animal protocols were conducted in accordance with the Kyoto Pharmaceutical University Guidelines for Animal Experimentation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kobuchi, S., Ito, Y. & Sakaeda, T. Population Pharmacokinetic–Pharmacodynamic Modeling of 5-Fluorouracil for Toxicities in Rats. Eur J Drug Metab Pharmacokinet 42, 707–718 (2017). https://doi.org/10.1007/s13318-016-0389-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-016-0389-3