Abstract
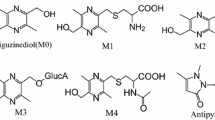
This study was to compare pharmacokinetics and bile transformation of R-enantiomer bambuterol with its racemate. Pharmacokinetics of R-enantiomer was investigated after single-dose intravenous and three doses of oral administration to rats and beagle dogs. To compare the pharmacokinetics with racemic bambuterol, the same oral doses of racemic bambuterol were also administrated; the blood and bile samples were collected by cannulation. A validated LC–MS/MS method was used to assess the level of bambuterol in plasma and bile. After single intravenous administration, no significant differences were observed between the two drugs in pharmacokinetic data. After oral dosing of R-bambuterol, the AUCs of R-enantiomer presented linear correlation. After same oral dosing of R-enantiomer and its racemate, all the pharmacokinetic parameters were equivalent. However, the clearance and apparent distribution had different results due to species and administration route difference. The bile transformation of these two compounds was similar and implicated that liver transformation accounted for the major metabolism of them. The bioavailability of R-enantiomer and racemate were comparative and relatively high in beagle dogs. Thus, R-enantiomer had a comparative pharmacokinetic profile and bile transformation with racemic bambuterol in rats and beagle dogs. These findings provided references for further clinical study.




Similar content being viewed by others
References
Bi HC et al (2008) Preclinical factors affecting the pharmacokinetic behaviour of tanshinone IIA, an investigational new drug isolated from Salvia miltiorrhiza for the treatment of ischaemic heart diseases. Xenobiotica 38:185–222. doi:10.1080/00498250701767675
Borgstrom L, Nyberg L, Jonsson S, Lindberg C, Paulson J (1989) Pharmacokinetic evaluation in man of terbutaline given as separate enantiomers and as the racemate. Br J Clin Pharmacol 27:49–56
Bosak A, Gazic I, Vinkovic V, Kovarik Z (2008) Stereoselective inhibition of human, mouse, and horse cholinesterases by bambuterol enantiomers. Chem Biol Interact 175:192–195. doi:10.1016/j.cbi.2008.04.050
Boulton DW, Fawcett JP (1997) Pharmacokinetics and pharmacodynamics of single oral doses of albuterol and its enantiomers in humans. Clin Pharmacol Ther 62:138–144. doi:10.1016/S0009-9236(97)90061-8
Cazzola M, Calderaro F, Califano C, Di Pema F, Vinciguerra A, Donner CF, Matera MG (1999) Oral bambuterol compared to inhaled salmeterol in patients with partially reversible chronic obstructive pulmonary disease. Eur J Clin Pharmacol 54:829–833
Fugleholm AM, Ibsen TB, Laxmyr L, Svendsen UG (1993) Therapeutic equivalence between bambuterol, 10 mg once daily, and terbutaline controlled release, 5 mg twice daily, in mild to moderate asthma. Eur Respir J 6:1474–1478
Gazic I, Bosak A, Sinko G, Vinkovic V, Kovarik Z (2006) Preparative HPLC separation of bambuterol enantiomers and stereoselective inhibition of human cholinesterases. Anal Bioanal Chem 385:1513–1519. doi:10.1007/s00216-006-0566-3
Handley DA, McCullough JR, Crowther SD, Morley J (1998) Sympathomimetic enantiomers and asthma. Chirality 10:262–272. doi:10.1002/(SICI)1520-636X(1998)10:3<262:AID-CHIR9>3.0.CO;2-B
Jeppsson AB, Johansson U, Waldeck B (1984) Steric aspects of agonism and antagonism at beta-adrenoceptors: experiments with the enantiomers of terbutaline and pindolol. Acta pharmacologica et toxicologica 54:285–291
Luo W, Zhu L, Deng J, Liu A, Guo B, Tan W, Dai R (2010) Simultaneous analysis of bambuterol and its active metabolite terbutaline enantiomers in rat plasma by chiral liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 52:227–231. doi:10.1016/j.jpba.2009.12.020
McFadden ER Jr (1995) Perspectives in beta 2-agonist therapy: vox clamantis in deserto vel lux in tenebris? J Allergy Clin Immunol 95:641–651
Noe S, Bohler J, Keller E, Frahm AW (1998) Determination of prednisolone in serum: method development using solid-phase extraction and micellar electrokinetic chromatography. J Pharm Biomed Anal 18:471–476
Nyberg L, Rosenborg J, Weibull E, Jonsson S, Kennedy BM, Nilsson M (1998) Pharmacokinetics of bambuterol in healthy subjects. Br J Clin Pharmacol 45:471–478
Perrin-Fayolle M (1995) Salbutamol in the treatment of asthma. Lancet 346:1101
Persson G, Baas A, Knight A, Larsen B, Olsson H (1995) One month treatment with the once daily oral beta 2-agonist bambuterol in asthmatic patients. Eur Respir J 8:34–39
Sanjar S, Kristersson A, Mazzoni L, Morley J, Schaeublin E (1990) Increased airway reactivity in the guinea-pig follows exposure to intravenous isoprenaline. J Physiol 425:43–54
Sitar DS, Warren CP, Aoki FY (1992) Pharmacokinetics and pharmacodynamics of bambuterol, a long-acting bronchodilator pro-drug of terbutaline, in young and elderly patients with asthma. Clin Pharmacol Ther 52:297–306
Testa B (2004) Prodrug research: futile or fertile? Biochem Pharmacol 68:2097–2106. doi:10.1016/j.bcp.2004.07.005
Tunek A, Svensson LA (1988) Bambuterol, a carbamate ester prodrug of terbutaline, as inhibitor of cholinesterases in human blood. Drug Metab Dispos 16:759–764
Waldeck B (2002) Beta-adrenoceptor agonists and asthma—100 years of development. Eur J Pharmacol 445:1–12
Walle UK, Pesola GR, Walle T (1993) Stereoselective sulphate conjugation of salbutamol in humans: comparison of hepatic, intestinal and platelet activity. Br J Clin Pharmacol 35:413–418
Ward JK, Dow J, Dallow N, Eynott P, Milleri S, Ventresca GP (2000) Enantiomeric disposition of inhaled, intravenous and oral racemic-salbutamol in man—no evidence of enantioselective lung metabolism. Br J Clin Pharmacol 49:15–22
Acknowledgments
This work was supported in part by National Natural Science Foundation of China (No. 31300774) and the Fundamental Research Funds for the Central Universities (No. 2013ZM0067).
Conflict of interest
The authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, S., Hu, Cy., He, My. et al. Comparative pharmacokinetics and bile transformation of R-enantiomer and racemic bambuterol after single-dose intravenous, oral administration in rats and beagle dogs. Eur J Drug Metab Pharmacokinet 40, 453–460 (2015). https://doi.org/10.1007/s13318-014-0228-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-014-0228-3