Abstract
Background
The pentavalent vaccine Pentavac was officially introduced in the Iranian National Immunization Plan in November, 2014.
Objective
To compare the immunogenicity and safety of Pentavac vaccine (Serum Institute of India Ltd.) with two other pentavalent vaccines available in Iran, i.e., Pentabio (PT Bio Farma (Persero)) and Shan 5 (Shantha Biotechnics Ltd.).
Design
Randomized, phase III study.
Participants
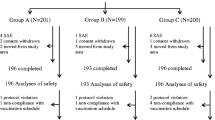
900 infants attending the study sites to receive the vaccine at 2, 4, and 6 months of age.
Intervention
Infants were randomly assigned to one of the Pentavac, Pentabio, and Shan 5 vaccine groups.
Outcomes
The antibody titers were measured against five antigens, diphtheria, tetanus, pertussis, Haemophilus influenzae B, and hepatitis B before receiving the first dose and one month after the last dose. The adverse events following vaccination after each dose were recorded in the adverse events diary.
Results
All vaccines showed similar immunogenicity against four of the five antigens except pertussis. While vaccination with Shan 5 resulted in the highest immunogenicity against pertussis, Pentabio was significantly lower than the other two vaccines (P<0.001). The incidence of local adverse events significantly differed among the three vaccine brands (P<0.001), but the incidence of most of the evaluated systemic adverse events was similar (P>0.05).
Conclusion
Pentavac and Shan 5 had similar immunogenicity, the former having better immunogenicity against pertussis than Pentabio. Pentavac and Pentabio had a comparable safety profile.
Similar content being viewed by others

References
Gandhi DJ, Dhaded SM, Ravi MD, et al. Safety, immune lot-to-lot consistency and non-inferiority of a fully liquid pentavalent DTwp-HepB-Hib vaccine in healthy Indian toddlers and infants. Hum Vaccin Immunother. 2016; 12:946–54.
Arístegui J, Usonis V, Coovadia H, et al. Facilitating the WHO expanded program of immunization: The clinical profile of a combined diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenza type b vaccine. Int J Infect Dis. 2003;7:143–51.
Karami M, Ameri P, Bathaei J, et al. Adverse events following immunization with pentavalent vaccine: Experiences of newly introduced vaccine in Iran. BMC Immunology. 2017;18:42.
Arjmand R., Gholami M., Shirvani F, et al. Study of serologic response rate to pertussis after administration of the third dose of pentavalent vaccine in children 12 months old in Karaj city, Iran. Int J Pediatr. 2018;6:7023–31.
Arjmand R, Golami M, Shirvani F, et al. Hepatitis B seroconversion rate after primary immunization series with newly introduced pentavalent vaccine: A report of local study in Alborz province, Iran, 2016. Arch Pediatr Infect Dis. 2019;7:e83565.
Rusmil K, Gunardi H, Fadlyana E, et al. The immuno-genicity, safety, and consistency of an Indonesia combined DTP-HB-Hib vaccine in expanded program on immuni-zation schedule. BMC Pediatr. 2015;15:219.
Rao R, Dhingra MS, Bavdekar S, et al. A comparison of immunogenicity and safety of indigenously developed liquid (DTwPHB-Hib) pentavalent combination vaccine (SHAN 5) with Easyfive (Liq) and Tritanrix + Hiberix (Lyo) in Indian infants administered according to the EPI schedule. Hum Vaccin. 2009;5:425–9.
Bachtiar N, Rusmil K, Sudigdoadi S, et al. The immunogenicity and safety of the new, Indonesian DTwP-HB-Hib vaccine compared to the DTwP/HB vaccine given with the Hib vaccine. PI. 2017:57:129–7.
Sharma HJ, Yadav S, Lalwani SK, et al. Immunogenicity and safety of an indigenously manufactured reconstituted pentavalent (DTwP-HBV+Hib) vaccine in comparison with a foreign competitor following primary and booster immunization in Indian children. Hum Vaccin. 2011;7:451–7.
Chatterjee S, Rego SJ, D’Souza F, et al. The immunogenicity and safety of a reduced PRP-content DTPw-HBV/Hib vaccine when administered according to the accelerated EPI schedule. BMC Infect Dis. 2010; 10:298.
Ali SS, Chandrashekar SR, Singh M, et al. A multicenter, prospective, open-label, non-comparative study to evaluate the immunogenicity and tolerance of a new, fully liquid pentavalent vaccine (DTwP-HepB-Hib vaccine). Hum Vaccine. 2007; 3:116–20.
Bavdekar SB, Maiya PP, Subba Rao SD, et al. Immuno-genicity and safety of combined diphtheria tetanus whole cell pertussis hepatitis B/Haemophilus influenzae type b vaccine in Indian infants. Indian Pediatr. 2007;44:505–10.
Prequalified vaccines. WHO — Prequalification of Medical Products (IVDs, Medicines, Vaccines. Accessed February 23, 2021. Available from: https://extranet.who.int/pqweb/vaccines/prequalified-vaccines/
Saffar MJ, Ajami A, Khalilian AR, et al. Pertussis seroimmunity among mother-infant pairs and infant immune response to pertussis vaccination. Indian Pediatr. 2007;44: 916–18.
Eregowda A, Lalwani S, Chatterjee S, et al. A phase III single arm, multicenter, open-label study to assess the immunogenicity and tolerability of a pentavalent DTwP-HepB-Hib vaccine in Indian infants. Hum Vacc Immunother. 2013; 9: 1903–9.
Sharma H, Yadav S, Lalwani S, et al. A phase III randomized, controlled study to assess the immunogenicity and tolera-bility of DTPw—HBV—Hib, a liquid pentavalent vaccine in Indian infants. Vaccine. 2011;29:2359–64.
Edwards KM, Berbers GA. Immune responses to pertussis vaccines and disease. J Infect Dis. 2014;209:S10–5.
Bar-On ES, Goldberg E, Fraser A, et al. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diph-theria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst Rev. 2009:CD005530.
Dalvi S, Kulkarni PS, Phadke MA, et al. A comparative clinical study to assess safety and reactogenicity of a DTwP-Hep B+Hib vaccine. Human Vacc Immunother. 2015;11:901–7.
Funding
Funding: Elite Researcher Grant Committee award (No. 940821) from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran. Sanofi Company, India, and Bio Farma, Indonesia provided the Shan 5 and Pentabio vaccines on a complimentary basis.
Author information
Authors and Affiliations
Contributions
Contributors: All authors approved the final version of manuscript, and are accountable for all aspects related to the study.
Corresponding author
Ethics declarations
Ethics clearance: National Institutes for Medical Research Development; No. IR.NIMAD.REC.1395.002 dated: 4 April 2016.
Competing interest: None stated.
Rights and permissions
About this article
Cite this article
Tabatabaei, S.R., Karimi, A., Zahraei, S.M. et al. Immunogenicity and Safety of Three WHO Prequalified (DTwP -HB-Hib) Pentavalent Combination Vaccines Administered As Per Iranian National Immunization Plan in Iranian Infants: A Randomized, Phase III Study. Indian Pediatr 58, 1131–1135 (2021). https://doi.org/10.1007/s13312-021-2393-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-021-2393-1