Summary
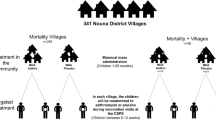
A group of American researchers funded by the Bill and Melinda Gates Foundation examined the effect of mass administration of azithromycin, on mortality in pre-school children. This was done through a community-based randomized controlled trial (RCT) designated MORDOR-I, conducted in Malawi, Niger, and Tanzania [1]. MORDOR is an acronym for the French title of the study. Community clusters of children (1 month to 5-year-old) were randomized to receive either azithromycin (single dose 20 mg/kg, administered twice a year for 2 years) or identical placebo (in the same dosage schedule). The overall mortality rate (expressed as deaths per 1000 person years) was 13.5% lower in the treatment arm, with 95% confidence interval 6.7% to 19.8% (hence statistically significant). However, detailed analysis showed that only communities in Niger had statistically significant mortality reduction to the extent of 18% (95% CI 10%, 25.5%), whereas those in Malawi and Tanzania did not. Thus, the overall mortality reduction was largely due to the reduction in Niger. This significant intercountry difference was partly attributed to higher baseline mortality in Niger, and stronger effect of mass azithromycin administration in such settings [2]. The investigators then evaluated the administration of two doses of azithromycin (6 months apart) in children from both groups of communities in Niger only. Thus, communities in the original Azithromycin group (in MORDOR-I) received a total of 6 doses, whereas those in the original placebo group received 2 doses. This part of the study has been designated MORDOR-II [3], and is examined in detail here. Communities in Malawi and Tanzania that did not show mortality decline were not evaluated any further.
The primary outcome in MORDOR-II [3] was the same as in MORDOR-I viz. all-cause mortality at the community level. Secondary outcomes included intra-group comparison of mortality. Although safety data were mentioned in the manuscript [3], the data were not presented. The results showed a comparable mortality rate (expressed as deaths per 1000 person-years) among children who received 6 doses of azithromycin over three years versus those who received 2 doses over 1 year. In contrast, the mortality after administration of 2 doses and 4 doses of azithromycin (versus similar doses of placebo) was 16.0% and 20.3% lower respectively, in the azithromycin group. Intra-group comparison showed that mortality in the original placebo group was 26.3 at the end of year 1 of MORDOR-I, 28.0 at the end of year 2 of MORDOR-I, and 24.0 at the end of MORDOR-II. This translated to an overall (statistically significant) 13.5% reduction in mortality between pre-MORDOR-I and post-MORDOR-II. In contrast, the intra-group comparison in the azithromycin group showed a 3.6% higher mortality after MORDOR-II, compared to before MORDOR-I (although the difference was not statistically significant). The authors reiterated their original conclusion that mass administration of azithromycin reduced mortality among pre-school children in Niger [1,3], and additional administration of two doses did not appear to wane this effect. However, there was no additional benefit on mortality with the third year of mass azithromycin administration.
Similar content being viewed by others
References
Keenan JD, Bailey RL, West SK, Arzika AM, Hart J, Weaver J, et al. Azithromycin to reduce childhood mortality in Sub-Saharan Africa. N Engl J Med. 2018;37: 1583–92.
Oron AP, Burstein R, Mercer LD, Arzika AM, Kalua K, Mrango Z, et al. Effect modification by baseline mortality in the MORDOR Azithromycin trial. Am J Trop Med Hyg. 2019 Feb 7. (Epub ahead of print).
Keenan JD, Arzika AM, Maliki R, Boubacar N, Elh Adamou S, Moussa Ali M, et al. Longer-term assessment of Azithromycin for reducing childhood mortality in Africa. N Engl J Med. 2019;380:2207–14.
Lund M, Pasternak B, Davidsen RB, Feenstra B, Krogh C, Diaz LJ, et al. Use of macrolides in mother and child and risk of infantile hypertrophic pyloric stenosis: nationwide cohort study. BMJ. 2014;348:1908.
Svanstrom H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704–12.
Gorelik E, Masarwa R, Perlman A, Rotshild V, Muszkat M, Matok I. Systematic review, meta-analysis, and network meta-analysis of the cardiovascular safety of macrolides. Antimicrob Agents Chemother. 2018; 62: e00438–18.
Xu P, Zeng L, Xiong T, Choonara I, Qazi S, Zhang L. Safety of azithromycin in pediatrics: A systematic review protocol. BMJ Pediatr Open. 2019;3: e000469.
Oldenburg CE, Arzika AM, Maliki R, Kane MS, Lebas E, Ray KJ, et al. Safety of azithromycin in infants under six months of age in Niger: A community randomized trial. PLoS Negl Trop Dis. 2018;12: e0006950.
Hansen MP, Scott AM, McCullough A, Thorning S, Aronson JK, Beller EM, et al. Adverse events in people taking macrolide antibiotics versus placebo for any indication. Cochrane Database Syst Rev. 2019;1: CD011825.
Porco TC, Hart J, Arzika AM, Weaver J, Kalua K, Mrango Z, et al. Mass oral azithromycin for childhood mortality: timing of death after distribution in the MORDOR trial. Clin Infect Dis. 2019;68:2114–16.
O’Brien KS, Emerson P, Hooper PJ, Reingold AL, Dennis EG, Keenan JD, et al. Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review. Lancet Infect Dis. 2019;19:e14–e5.
See CW, O’Brien KS, Keenan JD, Stoller NE, Gaynor BD, Porco TC, et al. The effect of mass Azithromycin distribution on childhood mortality: Beliefs and estimates of efficacy. Am J Trop Med Hyg. 2015; 93:1106–9
Arzika AM, Maliki R, Boubacar N, Kane S, Cotter SY, Lebas E, et al. Biannual mass Azithromycin distributions and malaria parasitemia in pre-school children in Niger: A cluster-randomized, placebo-controlled trial. PLoS Med. 2019;16: e1002835.
Doan T, Hinterwirth A, Worden L, Arzika AM, Maliki R, Abdou A, Kane S, et al. Gut microbiome alteration in MORDOR I: a community-randomized trial of mass azithromycin distribution. Nat Med. 2019. doi: https://doi.org/10.1038/s41591-019-0533-0
Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The immunomodulatory effects of macrolides-a systematic review of the underlying mechanisms. Front Immunol. 2018;9:302.
Tam CC, Offeddu V, Lim JM, Voo TC. One drug to treat them all: Ethical implications of the MORDOR trial of mass antibiotic administration to reduce child mortality. J Glob Health. 2019;1:010305.
Cutler T, Jannat-Khah D, Evans A. Azithromycin and childhood mortality in Africa. N Engl J Med. 2018;379:1382–84.
Sharma P, Kumari B, Dahiya S, Kulsum U, Kumar S, Manral N, et al. Azithromycin resistance mechanisms in typhoidal salmonellae in India: A 25 years analysis. Indian J Med Res. 2019;149:404–11.
Makkar A, Gupta S, Khan ID, Gupta RM, Rajmohan KS, Chopra H, et al. Epidemiological profile and antimicrobial resistance pattern of enteric fever in a tertiary care hospital of North India — a seven-year ambispective study. Acta Medica. 2018;61:125–30.
Sharma P, Dahiya S, Manral N, Kumari B, Kumar S, Pandey S, et al. Changing trends of culture-positive typhoid fever and antimicrobial susceptibility in a tertiary care North Indian hospital over the last decade. Indian J Med Microbiol 2018; 36:70–76.
References
UNICEF. Monitoring the situation of children and women. India (IND) — Demographics, Health & Infant Mortality — UNICEF DATA. Available from: https://data.unicef.org/country/ind/. Accessed August 22, 2019.
Liu Li, Chu Y, Oza S, Hogan D, Perin J, Bassani DG, et al. National, regional, and state-level all-cause and casue-specific under 5 mortality in India 2000–15: a systematic analysis with implications for the Sustainable Developmental Goals. Lancet Glob Health. 2019;7: e721–34.
Mejia-Guevara I, Zuo W, Bendavid E, Li N, Tuljapurkar S. Age distribution, trends, and forecasts of under-5 mortality in 31 sub-Saharan African countries: A modeling study. PLoS Med. 2019;16:e1002757.
Patel SR, Arti S, Pratap CB, Nath G. Drug resistance pattern in the recent isolates of S. typhi with special reference to cephalosporin and azithromycin in the Gangetic plain. J Clin Diagn Res. 2017;11:DM01–03.
Rasheed MK, Hasan SS, Babar Z, Ahmed SI. Extensively drug-resistant typhoid fever in Pakistan. Lancet Infect Dis. 2019;19:242–3.
Ghosh R, Uppal B, Aggarwal P, Chakrabarti A, Jha AK. Increasing antimicrobial resistance of Campylobacter jejuni isolated from pediatric diarrhoea cases in a tertiary care hospital of New Delhi, India. J Clin Diagn Res. 2013;7: 247–9.
Acknowledgement: Dr Melody Baruah, Consultant Microbiologist, Health City Hospital, Guwahati, India for the local azithromycin sensitivity results.
Funding
Funding: None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Extendibility: Can the results of MORDOR-I and MORDOR-II be applied in any setting outside Niger? Although the significant percentage reduction in mortality is impressive, the absolute reduction of 5 deaths per 1000 person-years [17], necessitates that 200 children be treated for at least one year, to prevent one death. This number-needed-to-treat is 10000 for Tanzania [17]. Viewed in this context, it is clear that individual settings (in different countries, or perhaps even within the same country) have to be examined very carefully before considering any policy of mass azithromycin administration.
Conclusion: Although India does not use mass azithromycin administration for trachoma control, and based on the data presented, there is no reason to consider this intervention in any part of the country, irrespective of the baseline childhood mortality. This is especially because, currently azithromycin resistance among typhoidal and non-typhoidal Salmonella is fairly low [18-20], and disturbing this can have serious consequences in the future.
Competing Interest: None stated.
Rights and permissions
About this article
Cite this article
Mathew, J.L., Das, R. Mass Administration of Azithromycin to Prevent Pre-school Childhood Mortality: Boon or Bane?. Indian Pediatr 56, 767–771 (2019). https://doi.org/10.1007/s13312-019-1633-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13312-019-1633-0