Abstract
Ocrelizumab is a recombinant humanized monoclonal antibody selectively targeting CD20-expressing B cells. The effect of ocrelizumab on primary progressive multiple sclerosis (PPMS) has been evaluated during phase 3 trials that enrolled patients under 55 years with a maximum Expanded Disability Status Scale (EDSS) of 6.5. However, little is known on older disabled patients with longer disease duration. We aimed to assess the clinical effectiveness of ocrelizumab in PPMS patients out of the ORATORIO eligibility criteria. This multicenter retrospective study collected data about the effectiveness of ocrelizumab in PPMS patients who received treatment between May 2017 and June 2022 in the Italian MS centers contributing to the Italian MS Registry who adhered to the Compassionate Use Program. The confirmed EDSS worsening (CEW) (defined as either a ≥ 1-point or ≥ 2-point increase in EDSS score from baseline that was confirmed at T12 and T24) was calculated. At the date of data extraction, out of 887 PPMS patients who had received ocrelizumab, 589 (mean age 49.7 ± 10.7 years, 242 (41.1%) females) were enrolled. The mean follow-up period was 41.3 ± 12.3 months. A total of 149 (25.3%) received ocrelizumab according to the ORATORIO criteria (ORATORIO group) and 440 (74.7%) outside the ORATORIO criteria (non-ORATORIO group). No differences in terms of cumulative probabilities of 12 and 24 months of CEW of ≤ 1 point were found between ORATORIO and non-ORATORIO groups. Cox regression analyses showed that age older than 65 years (HR 2.51, 25% CI 1.07–3.65; p = 0.01) was associated with higher risk of CEW at 24 months. Patients not responding to ORATORIO criteria for reimbursability may benefit from ocrelizumab treatment, as disease activity, disease duration, and EDSS seem to not impact the disability outcome. Our results may suggest to extend the possible use of this powerful agent in selected patients under the age of 65 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic, highly complex, inflammatory, and degenerative demyelinating disease of the central nervous system (CNS) causing neurological deficits referable to damage to the spinal cord, brainstem, optic nerves, cerebellum, and cerebrum [1,2,3]. Primary progressive MS (PPMS) is a relatively rare form of MS, accounting for approximately 10–15% of MS patients and it is characterized by a progressive course from disease onset with or without superimposed discrete clinical attacks or relapses [4,5,6]. According to several studies, PPMS patients typically exhibit a disabling course from symptom onset with a higher proportion of patients presenting at onset with motor impairment, cerebellar ataxia, and brainstem symptoms than relapsing-onset patients [6,7,8]. According to a recent classification, progressive MS form is further categorized according to the presence of disease activity in “active” and “non-active” [9, 10]. Several treatments, including therapies approved for the treatment of relapsing forms of MS such as interferons, fingolimod, natalizumab, and alemtuzumab, demonstrated limited effect on reducing the disability progression in patients with PPMS [11, 12]. Currently, PPMS remains a high disabling condition with very high unmet medical need.
Ocrelizumab is a recombinant humanized monoclonal antibody that selectively targets CD20-expressing B cells. CD20 is expressed on the cell surface of the pre-B cells and mature and memory B cells but not on lymphoid stem cells and plasma cells. Ocrelizumab is able to selectively deplete CD20-expressing B cells, however, not affecting the B cell reconstitution and pre-existing humoral immunity, and preserving the innate immunity and the total T cell numbers [13, 14].
In the ORATORIO trial (Study WA25046), a randomized, double-blind, placebo-controlled clinical trial in patients with PPMS, patients treated with ocrelizumab showed a significant reduction in the risk of progression of clinical disability (measured by the Expanded Disability Status Scale [EDSS]) sustained for at least 12 weeks by 24% and for at least 24 weeks by 25% compared with placebo. In another analysis, 42.7% of patients treated with ocrelizumab had no evidence of progression compared to 29.1% of patients treated with placebo 120 [15].
Since 2017, in Italy, ocrelizumab has been provided under Compassionate Use Program (CUP) (MA30130) for subjects who have been diagnosed of PPMS fulfilling the inclusion criteria indicated by the protocol (diagnosis of PPMS according to Mc Donald criteria) [1, 16]. In January 2018, the European Medicines Agency (EMA) authorized ocrelizumab for the treatment of adult patients with relapsing MS showing clinical or imaging feature characteristic of disease activity and with early PPMS in terms of disease duration and disability level, and with active disease defined by clinical and MRI features [17].
However, PPMS patients treated with ocrelizumab under CUP not fulfilling the ORATORIO criteria but reporting benefits from the ocrelizumab therapy were allowed to continue the treatment in the Italian MA30130 program according to the clinical judgement.
The aim of this retrospective multicenter study was to assess clinical efficacy of ocrelizumab in a population of PPMS patients receiving this treatment under CUP not satisfying the ORATORIO eligibility criteria compared to those patients responding to the labeled criteria of the ORATORIO [15, 16].
Methods
Study Population
This multicenter retrospective study is based on prospectively collected data about the effectiveness of ocrelizumab in PPMS patients who received treatment between May 2017 and June 2022 in all Italian MS centers contributing to the Italian MS Registry. Data are collected from two populations of PPMS patients treated with ocrelizumab: 1. those patients who received ocrelizumab treatment in the CUP program and not responding to the ORATORIO criteria [1, 15, 16]. These data were obtained by the Italian participating centers to CUP and stored in a repository collecting general standard information of patients included in the MA30130 program [16]; 2. those patients who started their therapy under CUP and kept on being treated with ocrelizumab after its approval and according to the labeled criteria for PPMS [16]. Data of this group of patients have been extracted by a secondary repository which is the Italian MS Registry.
The study was approved by the Policlinico-Vittorio Emanuele (Catania, Italy; cod 54/2021/PO) Ethics Committee. Ethical committee approval was also obtained from each individual participating center.
The inclusion criteria were the following: adult age; ability to provide written informed consent and to be compliant with the requirements regarding the schedule of treatment and all related treatment procedures; diagnosis of PPMS in accordance with the revised McDonald criteria (2017) [3]; at least 4 treatment courses of ocrelizumab and at least 3 EDSS evaluations.
The ORATORIO eligibility criteria for ocrelizumab treatment included the following: an age of 18 to 55 years, a score on EDSS of 3.0 to 6.5, a disease duration less than 15 years in patients with an EDSS score of more than 5.0 at screening or less than 10 years in patients with an EDSS score of 5.0 or less [15]. According to the presence of ORATORIO eligibility criteria, patients were divided in ORATORIO and non-ORATORIO groups. Moreover, we further stratified patients in the non-ORATORIO group according to the age (≤ 55, 56–64 and ≥ 65 years), EDSS (≤ 6.5 and > 6.5), and disease duration (≤ 10–15 and > 10–15 years, according to the EDSS).
Outcomes
In order to evaluate the disability progression during ocrelizumab treatment, EDSS evaluations were acquired at baseline (before ocrelizumab initiation), at 12 (T12) months, and 24 (T24) months after ocrelizumab initiation.
We stratified the cohort according to the presence of disease activity defined by the finding of active MS disease within the 24 months before starting ocrelizumab of clinical relapses and/or MRI activity [10]. Imaging features of inflammatory MRI activity were the following: at least one contrast-enhancing T1 lesion (CELs) or the development of at least 1 new or enlarging T2 lesions in comparison to the previous MRI [10].
The confirmed EDSS worsening (CEW) (defined as either a ≥ 1-point or ≥ 2-point increase in EDSS from baseline that was confirmed at T12 and T24) and the progression index (PI) (disability grade divided by duration of the disease) were calculated [18, 19].
Statistical Analysis
Statistical analysis was carried out using the statistic package STATA 16.1. Normal distribution was tested by the Shapiro–Wilk test. Continuous variables were expressed by number of observations, mean, and standard deviation (SD). Categorical data were presented by absolute and relative frequencies (n and %) or contingency tables. In case of violation of the assumptions for F or t-tests, equivalent non-parametric statistics will be used. All demographical and clinical characteristics were compared between the two groups. The Spearman correlation coefficient (Rho) was used to evaluate the strength of correlations between the analyzed variables.
Analysis of variance (ANOVA) was also applied to test the main and interactive effects among different subgroups. The Bonferroni test was used to correct for multiple post hoc pairwise comparisons. Kaplan–Meier curves were used to estimate the cumulative risk of developing CEW of at least 1 point at 24 months (1-point CEW). The variables significantly (p < 0.15) related with time to CEW on univariate analysis were included in the multivariate model. Multivariable Cox proportional hazard models were used to identify demographic and clinical variables significantly and independently associated with the outcome (1-point CEW at 24 months). The Cox proportional hazard models were corrected for age, sex, disease duration, “active” MS disease (yes/no), and the number of ocrelizumab courses. The null hypothesis was rejected if p < 0.05 (also an indicator of statistical significance). The adjusted hazard ratios (HRs) and their 95% CI were used to interpret the final model. A two-sided P value of < 0.05 was considered statistically significant.
Results
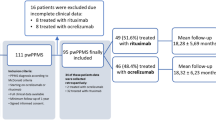
At the date of data extraction, out of 887 PPMS patients who had received ocrelizumab, 589 (mean age 49.7 ± 10.7 years, 242 [41.1%] females) fulfilled the inclusion criteria and were finally enrolled (Fig. 1). We found 149 (25.3%) received ocrelizumab according to the ORATORIO criteria (ORATORIO group) and 440 (74.7%) outside the ORATORIO criteria (non-ORATORIO group).
Patients’ selection flow chart. EDSS, Expanded Disability Status Scale; PPMS, primary progressive multiple sclerosis. The ORATORIO group includes patients fulfilling the ORATORIO eligibility criteria for ocrelizumab treatment (age of 18 to 55 years, EDSS of 3.0 to 6.5, disease duration less than 15 years in patients with an EDSS score of more than 5.0 at screening or less than 10 years in patients with an EDSS score of 5.0 or less [14]); non-ORATORIO includes patients not fulfilling the ORATORIO criteria
Among the patients in the non-ORATORIO group, 252 (57.3%) were older than 55 years, 126 (74.7%) had an EDSS higher than 6.5, and 185 (42%) had a disease duration longer than 10 or 15 years (according to EDSS) at the time of treatment initiation (Fig. 1).
The mean follow-up period was 41.3 ± 12.3 months. Demographic and clinical baseline characteristics are summarized in Table 1.
The proportion of patients with disease activity at the time of ocrelizumab initiation was similar between ORATORIO and non-ORATORIO groups (Table 1). In addition, in the non-ORATORIO group, a higher percentage of active patients was found in those with EDSS > 6.5 (33 [23.2%] versus 23 [7.3%], p < 0.001), while no differences were found in the other subgroups (see Supplementary Materials).
The cumulative probabilities of 12 and 24 months of CEW of ≤ 1 point were 22.1% and 22.8%, respectively, in the ORATORIO group, and 18.4% and 27.5%, respectively, in the non-ORATORIO group. The cumulative probabilities of 12 and 24 months of CEW of ≥ 2 points were 3.4% and 5.4%, respectively, in the ORATORIO group, and 3.4% and 5%, respectively, in the non-ORATORIO group (Table 2).
Among patients in the non-ORATORIO group, patients with age > 56 years exhibited higher values of 12 and 24 months of CEW of ≥ 1 point and 12 and 24 months of CEW of ≥ 2 points compared to those patients aged < 55 years (Table 3).
No significant differences in terms of 12 and 24 months of CEW of ≥ 1 point and 12 and 24 months of CEW of ≥ 2 points were found between patients stratified according to the EDSS and the disease duration at the time of ocrelizumab initiation (Tables 4 and 5).
Further stratification of patients aged > 55 years revealed that patients with age > 65 years at the time of ocrelizumab initiation showed significantly higher CEW of ≥ 1 point at 12 months and of CEW of ≥ 2 points at 12 and 24 months compared to those patients with age between 56 and 64 years and to those with age ≤ 55 years (Table 6).
No significant differences in terms of 1-point CEW at 24 months were found between patients stratified according to the presence of disease activity at the time of ocrelizumab initiation (p = 0.8 and p = 0.5 in ORATORIO and non-ORATORIO groups, respectively) (Fig. 2A). Particularly, in the non-ORATORIO group, proportion of patients who reached 1-point CEW at 24 months was similar between “active” and “non-active,” in each subgroup (Fig. 2B). Stratifying the age at the time of ocrelizumab initiation in three categories (≤ 55, 56–64, and ≥ 65 years), no significant differences were found in 1-point CEW at 24 months (Fig. 2C).
Proportion of patients who reached 1-point CEW at 24 months, stratified according to the presence of disease activity in ORATORIO and non-ORATORIO groups (A), in each non-ORATORIO subgroup (B), and in each age category (C). CEW, confirmed EDSS worsening of at least 1 point at 24 months; EDSS, Expanded Disability Status Scale
In addition, no differences in terms of PI at 12 and 24 months were found between the ORATORIO and non-ORATORIO group (Table 1).
The Cox proportional hazard model showed that age older than 65 years at the time of ocrelizumab initiation was independently associated with higher risk of CEW at 24 months (HR 2.51, 25% CI 1.07–3.65; p = 0.01). The results of the Cox regression analysis for time to CEW in all patients are illustrated in Fig. 3. The Kaplan–Meier–estimated cumulative risk of CEW was similar in ORATORIO and non-ORATORIO groups (p = 0.6) (Fig. 4A) and stratifying according to EDSS (p = 0.8) (Fig. 4B) and to disease duration (p = 0.8) (Fig. 4C). Age older than 65 years was significantly associated with a shorter time to reach CEW (p < 0.01) (Fig. 4D).
Kaplan–Meier curves for the time of reaching 1-point CEW at 24 months during treatment with ocrelizumab in the entire cohort (A), and in patients stratified according to EDSS (≤ 6.5 and > 6.5) (B), disease duration (≤ 15 or 10 and > 15 or 10 years) (C), and age (≤ 55, 56–64 and > 65 years) (D). CEW, confirmed EDSS worsening of at least 1 point at 24 months; EDSS, Expanded Disability Status Scale; DD, disease duration
Discussion
In this multicenter, observational study, ocrelizumab administrations showed similar effects on disability progression in both PPMS patients responding or not to the ORATORIO eligibility criteria.
Among patients who did not present the ORATORIO criteria, those with age > 65 years at the time of ocrelizumab initiation showed significantly higher CEW of ≥ 1 point at 12 months and of CEW of ≥ 2 points at 12 and 24 months compared to those patients with age between 56 and 64 years and with age ≤ 55 years. No significant differences in terms of 12 and 24 months of CEW of ≤ 1 point and 12 and 24 months of CEW of ≥ 2 points were found between patients stratified according to the EDSS and the disease duration at the time of ocrelizumab initiation.
The anti-CD20 monoclonal antibody ocrelizumab was the first disease-modifying therapy to be approved for treatment of PPMS. A previous phase 2–3 trial of rituximab (OLYMPUS), a chimeric monoclonal anti-CD20 antibody, in PPMS patients did not meet its primary efficacy endpoint; however, a subgroup analysis demonstrated that patients with age < 51 years showed delayed progression of disability [20]. Subsequently, the ORATORIO trial investigated the effect of ocrelizumab in patients’ PPMS and randomized 732 patients to treatment with either ocrelizumab or placebo (2:1) for 3 years. In this study, ocrelizumab was able to reduce the percentage of patients with 12-week confirmed EDSS disability progression (HR = 0.76; 95% CI, 0.59–0.98; p < 0.03) [15].
Later, real-world evidences have confirmed the effectiveness of ocrelizumab in reducing relapses, MRI activity, and slowing down the rate of progression in PPMS patients [21, 22], although another recent observational comparative study of a small cohort of 13 PPMS and 29 relapsing–remitting MS indicated that the effect of ocrelizumab on disability progression was more evident for relapsing–remitting MS [23].
In line with our results showing that patients older than 55 years had similar disability progression compared to younger ones, a recent retrospective real-world study demonstrated that among 56 patients older than the age of 55 at the time of ocrelizumab initiation, a high percentage of patients, about 60%, remained stable or improved after 2 years of ocrelizumab treatment [24].
On the other hand, we found that age older than 65 is associated to higher risk of disability progression. Indeed, age-related functional changes of the innate and adaptive immune, referred to as immunosenescence, with the resulting low-grade proinflammatory state (inflammaging), may impact the efficacy as well as the safety profile of current DMTs [25]. The immunosenescence can affect the T and B cells, monocytes and macrophages, microglia, dendritic cells, and natural killer cells, also inducing a reduction and functional alterations of the naïve B cell population of clonal expansion capabilities of memory cells and of antibody levels and antibody specificity [26, 27].
Moreover, several studies observed that old age in MS is associated to a greater multimorbidity risk and, in turn, the presence of comorbidities, such as cardiovascular diseases, is considered a risk factor for disability accumulation [28, 29]. Age-associated comorbidities may also influence the risk–benefit analysis for DMTs and be accompanied to reduced efficacy.
A recent meta-analysis of randomized, blinded clinical trials of MS DMTs against placebo or active comparator involving more than 28,000 MS patients revealed that the efficacy of immunomodulatory DMTs on disability progression strongly decreases with age, demonstrating a loss of efficacy at an average age of 53 [30]. Particularly, highly active drugs seemed to reduce their higher efficacy, as compared to low-efficacy drugs in patients aged 40.5 years and older [30].
Notably, current clinical trials have excluded patients over age 55, and thus, there are no data suggesting that DMTs are either effective in the elderly, especially in those without disease activity. Indeed, an age gap exists between the MS clinical trial and real-world populations due to the growing numbers of elderly people with MS [31]. This makes clinical trial results less applicable to the aging real-world MS population in terms of age and age-related changes in disease activity.
In our study, disease duration longer than 10–15 years and EDSS < 6.5 seemed to not affect the disability outcome. The evidence regarding the predictive power of disease duration on disease progression was mixed, and several studies have shown that disease duration did not consistently predict disability worsening, particularly in the long-term [32, 33].
Similarly, the prognostic limitations of the EDSS across several domains has been widely demonstrated [34]. Indeed, while lower EDSS values are mainly based on impairments detected by the neurologic examination, values higher than 4 are deeply influenced by walking disability. In addition, several studies suggested that EDSS scores of 6 and higher are less sensitive to change in progression of the disease [35, 36]. Furthermore, it should be noted that EDSS does not adequately gather the possible changes of cognitive function, upper extremity ability, and fatigue, which are demonstrated to be relevant predictors of long-term disease progression in MS [33].
In addition, as observed in a post hoc analysis of OPERA I and II trials on relapsing MS patients, sex did not affect progression outcomes in our cohort of patients [37]. On the contrary, another real-world study showed that male gender and longer follow-up period were independent predictors for disability progression in a cohort of 48 PPMS with a follow-up longer than 1 year [38].
Interestingly, the presence of disease activity at the time of treatment initiation seemed to not influence the outcome. This is apparently in contrast with the current literature showing that powerful therapies are more efficacious in reducing relapses in patients with active progressive MS [39]. However, subsequent analyses on ORATORIO dataset could not demonstrate significant differences regarding the response to ocrelizumab in active and non-active PPMS patients [14, 40]. Indeed, our current understanding of disease activity mainly focuses on inflammation-related relapses and/or MRI activity [10, 14], while to date, the effects of powerful DMTs on disability accumulation without immunological activity (also called “progression independent of relapse activity” [PIRA]) are less well known [41].
Our study has several limits. The retrospective design may have limited the statistical power of our results. Moreover, the use of EDSS as clinical endpoint may underestimate the possible worsening of disability progression in our cohort because of a low event rate and fluctuation in scores. Finally, our study did not investigate the safety profile of ocrelizumab in patients who did not fulfill the ORATORIO criteria; further analyses are required to characterize the risk of adverse events, including progressive multifocal leukoencephalopathy (PML) in this group of patients, particularly in the elderly. As the probability of active disease declines with age while the susceptibility to adverse events increases, the risks versus benefits of using ocrelizumab in the elderly should be verified in longitudinal studies.
In conclusion, our results showed that disease activity, disease duration, and EDSS at the time of ocrelizumab initiation seem to not impact the disability outcomes. Patients not responding to ORATORIO criteria for reimbursability may benefit from ocrelizumab treatment, thus suggesting to extend the possible use of this powerful agent in selected patients under the age of 65 years.
Availability of Data and Material
Dataset is available under reasonable request to the corresponding author.
References
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7.
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol. 2005;58(6):840–6.
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2017.
Chataway J. Evolving diagnostic criteria for multiple sclerosis. Lancet Neurol. 2018;17(2):118.
Ntranos A, Lublin F. Diagnostic criteria, classification and treatment goals in multiple sclerosis: the chronicles of time and space. Curr Neurol Neurosci Rep. 2016;16(10):90.
Hawker K. Primary progressive multiple sclerosis. Continuum (Minneap Minn). 2010;16(5 Multiple Sclerosis):211–25.
Harding KE, Wardle M, Moore P, Tomassini V, Pickersgill T, Ben-Shlomo Y, et al. Modelling the natural history of primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86(1):13–9.
Rocca MA, Sormani MP, Rovaris M, Caputo D, Ghezzi A, Montanari E, et al. Long-term disability progression in primary progressive multiple sclerosis: a 15-year study. Brain. 2017;140(11):2814–9.
Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5.
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–86.
Segal BM, Stuve O. Primary progressive multiple sclerosis–why we are failing. Lancet. 2016;387(10023):1032–4.
Kantarci O. Treatment of primary progressive multiple sclerosis. Semin Neurol. 2013;33(1):74–8.
Stahnke AM, Holt KM. Ocrelizumab: a new B-cell therapy for relapsing remitting and primary progressive multiple sclerosis. Ann Pharmacother. 2018;52(5):473–83.
Montalban X, Belachew S, Wolinsky JS. Ocrelizumab in primary progressive and relapsing multiple sclerosis. N Engl J Med. 2017;376(17):1694.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20.
Ufficiale G. https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2018-09-03&atto.codiceRedazionale=18A05713&elenco30giorni=false. Last Access 25 Jan 2023.
(EMA) EMA. Ocrevus, INN-ocrelizumab 2020 [Available from: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_it.pdf].
Trojano M, Butzkueven H, Kappos L, Wiendl H, Spelman T, Pellegrini F, et al. Natalizumab treatment shows low cumulative probabilities of confirmed disability worsening to EDSS milestones in the long-term setting. Mult Scler Relat Disord. 2018;24:11–9.
Cendrowski WS. Progression index and disability status in multiple sclerosis: a resurvey of 207 patients in central Poland. Schweiz Arch Neurol Psychiatr. 1986;137(4):5–13.
Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–71.
Montalban X, Matthews PM, Simpson A, Petrie JL, Sammon C, Ramagopalan S, et al. Real-world evaluation of ocrelizumab in multiple sclerosis: a systematic review. Ann Clin Transl Neurol. 2023;10(3):302–11.
Lanzillo R, Carotenuto A, Signoriello E, Iodice R, Miele G, Bisecco A, et al. Prognostic markers of ocrelizumab effectiveness in multiple sclerosis: a real world observational multicenter study. J Clin Med. 2022;11(8).
Rodriguez de Antonio LA, Cuberta-Gonzalez I, Garcia-Castanon I, Oreja-Guevara C. Relapsing-remitting and primary progressive multiple sclerosis treated with ocrelizumab: a comparative study. Mult Scler Relat Disord. 2023;69:104441.
Epstein S, Fong KT, De Jager PL, Levine L, Riley C, Wesley S, et al. Evaluation of ocrelizumab in older progressive multiple sclerosis patients. Mult Scler Relat Disord. 2021;55:103171.
Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, et al. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960.
Buffa S, Bulati M, Pellicano M, Dunn-Walters DK, Wu YC, Candore G, et al. B cell immunosenescence: different features of naive and memory B cells in elderly. Biogerontology. 2011;12(5):473–83.
Grebenciucova E, Berger JR. Immunosenescence: the role of aging in the predisposition to neuro-infectious complications arising from the treatment of multiple sclerosis. Curr Neurol Neurosci Rep. 2017;17(8):61.
Marrie RA, Rudick R, Horwitz R, Cutter G, Tyry T, Campagnolo D, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041–7.
Maric G, Pekmezovic T, Tamas O, Veselinovic N, Jovanovic A, Lalic K, et al. Impact of comorbidities on the disability progression in multiple sclerosis. Acta Neurol Scand. 2022;145(1):24–9.
Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–40.
Lavorgna L, Bonavita S, Ippolito D, Lanzillo R, Salemi G, Patti F, et al. Clinical and magnetic resonance imaging predictors of disease progression in multiple sclerosis: a nine-year follow-up study. Mult Scler. 2014;20(2):220–6.
Moccia M, Lanzillo R, Palladino R, Chang KC, Costabile T, Russo C, et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult Scler. 2016;22(5):659–67.
Cinar BP, Yorgun YG. What we learned from the history of multiple sclerosis measurement: Expanded Disability Status Scale. Noro Psikiyatr Ars. 2018;55(Suppl 1):S69–75.
Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:58.
Wang G, Cutter GR, Cofield SS, Lublin F, Wolinsky JS, Gustafson T, et al. Baseline EDSS proportions in MS clinical trials affect the overall outcome and power: a cautionary note. Mult Scler. 2017;23(7):982–7.
Turner B, Cree BAC, Kappos L, Montalban X, Papeix C, Wolinsky JS, et al. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J Neurol. 2019;266(5):1182–93.
Fernandez-Diaz E, Perez-Vicente JA, Villaverde-Gonzalez R, Berenguer-Ruiz L, Candeliere Merlicco A, Martinez-Navarro ML, et al. Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann Clin Transl Neurol. 2021;8(2):385–94.
Roos I, Leray E, Casey R, Horakova D, Havrdova E, Izquierdo G, et al. Effects of high- and low-efficacy therapy in secondary progressive multiple sclerosis. Neurology. 2021;97(9):e869–80.
Manouchehri N, Stuve O. Should ocrelizumab be used in non-active primary progressive multiple sclerosis? Time for a re-assessment. Ther Adv Neurol Disord. 2021;14:1756286421990500.
Lublin FD, Haring DA, Ganjgahi H, Ocampo A, Hatami F, Cuklina J, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145(9):3147–61.
Acknowledgements
Paolo Bellantonio, Giovanna De Luca, Francesca De Robertis, Franco Granella, Alice Laroni, Agostino Nozzolillo, Giuseppe Salemi, Marco Salvetti, Rocco Totaro, Marika Vianello, Mauro Zaffaroni.
Funding
Open access funding provided by Università degli Studi di Catania within the CRUI-CARE Agreement. This is an independent Italian, retrospective, multicenter study supported by Roche® unconditional grant (n. SL43058).
Author information
Authors and Affiliations
Consortia
Contributions
CGC and FP contributed to study concept and design, analysis and interpretation of data, and drafting of the manuscript. AB, VBM, FC, PC, CC, CrC, MD, MF, PI, RL, AL, Alu, SLF, GL, AGM, FM, MM, LM, CP, SR, ST, MT, and DV contributed to the acquisition of data, analysis and interpretation of data, and study supervision, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from all patients eligible for the study. This protocol, the Informed Consent Forms, any information to be given to the patient, and relevant supporting information, was approved by the IRB/EC of the coordinator center and by all the centers participating to the study.
Competing Interests
CGC has received grants for congress participation from Almirall, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme, and TEVA. AB received compensation for consulting services and participation in advisory board and travel grants from Almirall, Biogen, Novartis, Roche, Sanofi- Genzyme, and Merck-Serono. VBM declares no conflict of interest. MC received compensation for speaking activities, travel grant, and participation in advisory board from Biogen, Bristol-Myers Squibb, Merck-Serono, Novartis, Roche, and Sanofi-Genzyme. FC has received travel grants from Biogen, Merck, Sanofi-Genzyme, and Roche and research grants from Merck. PC received honoraria as speaker or travel grants to attend national and international conferences or consultation for advisory boards from Alexion, Almirall, Bayer Schering, Biogen, Cellgene-BMS, Merck-Serono, Teva, Roche, Novartis, Sanofi-Genzyme, and Janssen. She is the principal investigator in clinical trials for Roche, Sanofi-Genzyme, and Merck Serono. CC declares no conflict of interest. CrC declares no conflict of interest. MD declares no conflict of interest. MF is Editor-in-Chief of the Journal of Neurology; Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, and Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, and Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, and Sanofi-Genzyme; receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). PI has received honoraria for lecturing and participation in advisory boards, and/or travel expenses for attending congresses and meetings from Almirall, Biogen Idec, Merck Serono, Mylan, Novartis, Roche, Sanofi Genzyme, and TEVA. RL declares no conflict of interest. AL declares no conflict of interest. ALu has served as a Biogen, Bristol Myers Squibb, Merck Serono, Novartis, Roche, and Sanofi/Genzyme Advisory Board Member. She received congress and travel/accommodation expense compensations or speaker honoraria from Alexion, Biogen, Merck Serono, Novartis, Roche (2020), Sanofi/Genzyme, and Fondazione Italiana Sclerosi Multipla (FISM). Her institutions received research grants from Novartis and Sanofi/Genzyme. SLF has received honoraria for lecturing and participation in advisory boards, and/or travel expenses for attending congresses and meetings from Almirall, Biogen Idec, Merck Serono, Mylan, Novartis, Roche, Sanofi Genzyme, and TEVA. GL has received honoraria for lecturing and participation in advisory boards, and/or travel expenses for attending congresses and meetings from Almirall, Biogen Idec, Merck Serono, Mylan, Novartis, Roche, Sanofi Genzyme, and TEVA. AGM is an advisory board member of Biogen Idec, Genzyme, Merck-Serono, Novartis, Almirall, Alexion, and Roche and received honoraria for speaking or consultation fees from Alexion, Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi-Genzyme, Roche, Janssen, Viatris, and Bristol-Myers Squiibb. She is the principal investigator in clinical trials for Biogen Idec, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Merck Serono. FM received compensation for speaking activities, travel grant, and participation in advisory board from Biogen, Bristol-Myers Squibb, Novartis, Roche, Sanofi-Genzyme, and Merck-Serono. MM received compensation for consulting services, speaking activities, and participation in advisory board from Alexion, Almirall, Bayer, Biogen, Bristol-Myers Squibb, Celgene, CSL Behring, Novartis, Roche, Sanofi-Genzyme, Janssen, Merck-Serono, and Viatris; he received research support from Biogen, Merck-Serono, Novartis, and Roche. LM received compensation for speaking activities, travel grant, and participation in advisory board from Biogen, Bristol-Myers Squibb, Novartis, Roche, Sanofi-Genzyme, Merck-Serono, Biogen, and Alexion. CP received compensation for speaking activities, travel grant and participation in advisory board from Biogen, Bristol-Myers Squibb, Merck-Serono, Novartis, Roche, Sanofi-Genzyme. SR received compensation for speaking activities, travel grant, and participation in advisory board from Biogen, Bristol-Myers Squibb, Novartis, Roche, Sanofi-Genzyme, Merck-Serono, and Viatris. ST declares no conflict of interest. MT reported receiving speaker honoraria and research grants to her institution from and serving on advisory boards of Biogen, Merck Serono, and Novartis. DV declares no conflict of interest. FP has received honoraria for speaking activities by Almirall, Bayer Schering, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme, and TEVA; he also served as advisory board member from the following companies: Bayer Schering, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme, and TEVA; he was also funded by Pfizer and FISM for epidemiological studies; he received grants for congress participation from Almirall, Bayer Shering, Biogen Idec, Merck Serono, Novartis, Roche, Sanofi Genzyme, and TEVA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chisari, C.G., Bianco, A., Brescia Morra, V. et al. Effectiveness of Ocrelizumab in Primary Progressive Multiple Sclerosis: a Multicenter, Retrospective, Real-world Study (OPPORTUNITY). Neurotherapeutics 20, 1696–1706 (2023). https://doi.org/10.1007/s13311-023-01415-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-023-01415-y