Abstract
Multiple system atrophy (MSA) is a rare neurodegenerative disorder with unclear etiology, currently difficult and delayed diagnosis, and rapid progression, leading to disability and lethality within 6 to 9 years after symptom onset. The neuropathology of MSA classifies the disease in the group of a-synucleinopathies together with Parkinson’s disease and other Lewy body disorders, but features specific oligodendroglial inclusions, which are pathognomonic for MSA. MSA has no efficient therapy to date. Development of experimental models is crucial to elucidate the disease mechanisms in progression and to provide a tool for preclinical screening of putative therapies for MSA. In vitro and in vivo models, based on selective neurotoxicity, a-synuclein oligodendroglial overexpression, and strain-specific propagation of a-synuclein fibrils, have been developed, reflecting various facets of MSA pathology. Over the years, the continuous exchange from bench to bedside and backward has been crucial for the advancing of MSA modelling, elucidating MSA pathogenic pathways, and understanding the existing translational gap to successful clinical trials in MSA. The review discusses specifically advantages and limitations of the PLP-a-syn mouse model of MSA, which recapitulates motor and non-motor features of the human disease with underlying striatonigral degeneration, degeneration of autonomic centers, and sensitized olivopontocerebellar system, strikingly mirroring human MSA pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Multiple System Atrophy — Introduction
The term multiple system atrophy (MSA) was introduced in 1969 by Oppenheimer and Graham [1]. They observed overlapping clinical presentations in the syndromes of sporadic olivopontocerebellar atrophy (OPCA), striatonigral degeneration (SND), and Shy-Drager syndrome and therefore suggested the unifying diagnosis of MSA. The accuracy of this suggestion was confirmed 20 years later by the neuropathological observation of argyrophilic inclusion bodies with “tubular structure” in the oligodendrocytes of patients with different combinations of MSA syndromes. These oligodendroglial aggregates were named glial cytoplasmic inclusions (GCIs) [2]. Another 9 years were needed to identify filamentous alpha-synuclein (a-syn) as a component of GCIs linking MSA with Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) within the group of a-synucleinopathies [3,4,5].
Epidemiological Features and Natural History of MSA
MSA is a rare, rapidly progressive neurodegenerative disorder with the profile of an orphan disease. The usual symptom onset is in the fifth decade of life. Its incidence ranges between 0.1 and 2.4/100,000 per year increasing with age, and the estimated prevalence is 4.4/100,000 [6, 7]. Men and women are similarly affected. The disease duration after first diagnosis is 7.9 ± 2.8 years, i.e., much shorter than in PD [8]. The spectrum of symptoms in MSA includes parkinsonism, ataxia, autonomic dysfunction, and pyramidal signs in various combinations [9]. The studies on the natural history of the disease indicate that usually, non-motor symptoms are the first to be reported. These may include orthostatic hypotension, urogenital dysfunction, sleep, and respiratory disorder (stridor). The actual clinical diagnosis of MSA according to the current criteria is possible only after the onset of motor symptoms, which, based on the predominant syndrome, define the Parkinsonian variant (MSA-P) or the cerebellar variant (MSA-C) [10]. The clinical diagnosis can be set with different degrees of certainty (possible or probable), but the final diagnosis is currently possible only postmortem with the demonstration of GCIs in the brain. The diagnostic accuracy is often reduced by the overlapping symptoms with other disorders like PD, DLB, or progressive supranuclear palsy (PSP) [11]. The clinical decline of MSA patients is very rapid, and they may reach milestones of disability within a short period of about 5 years after the first diagnosis [8]. The devastating character of MSA is determined not only by its rash progression but also by the lack of efficient therapy. The symptomatic treatments have usually limited benefit and are unable to stop the progression of the degeneration [9].
In summary, MSA presents a serious medical and social problem with its delayed diagnosis according to the current criteria, low diagnostic accuracy, rapid progression, and early disability of the patients parallel to the lack of efficient therapy. For all these reasons, it is of paramount importance to understand the disease mechanisms and define molecular targets that may support an improved early diagnosis as well as serve as the key towards disease modification in MSA.
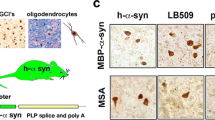
The Neuropathology of MSA — Milestones and Recent Discoveries
One of the main sources to get a glimpse into the disease mechanisms is usually the neuropathological examination of the brain. The neuropathological milestones of MSA include widespread pathognomonic a-syn-positive GCIs, selective neurodegeneration with SND and OPCA of various severity and combinations, and degeneration of autonomic CNS centers in the brainstem and spinal cord. In the postmortem brain, gliosis, myelin changes, and demyelination usually accompany the neurodegeneration [12,13,14,15,16,17]. Neuroinflammatory signatures in the MSA brain include microglial upregulation of toll-like receptor 4 (TLR4) [18], myeloperoxidase (MPO) [19], and inflammasome-related proteins like NLRP3, ASC, and caspase 1 [20]. In addition, the pro-inflammatory cytokines TNF-α, IL-1β, and Il-6 are found increased in the cerebrospinal fluid (CSF) of MSA patients [21]. Therefore, neuroinflammatory changes linked to microglial activation have been suggested as a possible player in MSA pathogenesis [22]. The myelin dysfunction with possible early relocation and accumulation of p25a/TPPP from the myelin sheaths to the oligodendroglial soma has indicated a possible primary oligodendrogliopathy [17, 23], which may be an early pathogenic event in the disease cascade. Finally, a specificity of a-synucleinopathy has been described at cellular and molecular level in MSA as compared to Lewy body (LB) disorders. A-syn in GCIs is rich in post-translational modifications like phosphorylation at Ser129 [24] and widespread nitration [25]. The initially shown disease-specific widespread ectopic aggregation of a-syn in oligodendrocytes in MSA is accompanied by neuronal cytoplasmic and nuclear a-syn inclusions, which structurally differ from LBs [26,27,28,29]. Recent findings have suggested a different structure of the a-syn fibrils in MSA with a different seeding profile as compared to other a-synucleinopathies [30, 31]. It is unclear yet whether the disease-specific a-syn strains are causative for the different a-synucleinopathies or rather represent a secondary event of specific misfolding within a different pathogenic environment. It is known that a-syn fibrils are not the only constituent of GCIs and the structure of these pathological aggregates includes a large number of other components [32]. Neuropathological analysis has suggested the disruption of the ubiquitin–proteasome system (UPS) and the autophagy-lysosomal pathway (ALP) in MSA [33,34,35], but it remains unclear whether these defects have a causative role in GCI formation or rather represent a consequence of the effects of misfolded a-syn on the function of UPS and ALP. Finally, it is still under debate whether the inclusion pathology in MSA plays a detrimental role in the disease pathogenesis or represents a rescue mechanism of the cells and acts as a “trash bin” for the misfolded proteins accumulating in the cell. The origin of a-syn in oligodendrocytes of MSA is largely uncertain. Earlier studies have claimed that a-syn is a neuronal protein, which is not expressed in mature oligodendroglia [36]. However, laser dissection of oligodendroglia from MSA and control brains has suggested that MSA oligodendrocytes show a tendency to express more SNCA mRNA than control oligodendrocytes [37], supporting a possible oligodendroglial a-synucleinopathy. Oligodendroglial progenitor cells (OPCs) are known to express SNCA mRNA, but maturation to oligodendrocytes is associated with physiological decline of a-syn expression [38]. A postmortem analysis in MSA patients has proposed an increased number of striatal OPCs [39], which may indicate a dysfunctional maturation of the oligodendroglial lineage in MSA. Although the density of OPCs has been identified increased in the white matter of the MSA brain, it has been linked to demyelination, but not to accumulation of a-syn in OPCs [40].
The findings in minimal change MSA cases, in which the disease is characterized by widespread GCIs, restricted neuronal loss, and short duration, have suggested that a-syn-associated oligodendroglial pathology may lead to neuronal dysfunction sufficient to cause clinical symptoms before overt neuronal loss in MSA [41,42,43].
Finally, the neuropathological examination of peripheral and autonomic nerves in MSA has evidenced the presence of phosphorylated S129 a-syn and a-syn oligomers with nerve fiber degeneration in the skin [44]. Importantly, Schwann cells in cranial and spinal nerves, spinal and sympathetic ganglia, but only rarely in visceral nerves have been shown to form filamentous a-syn inclusions of phosphorylated a-syn in MSA [45]. To that, myenteric neurons in MSA have been reported to present with shrinkage of the soma without phosphorylated a-syn accumulation [46]. Although the number of studies on the peripheral a-synucleinopathy in MSA is limited, the notion is that Schwann cell synucleinopathy may precede the nerve dysfunction similar to the a-syn oligodendroglial pathology in the CNS.
Evidence on the Cause of MSA so far — the Possible Interplay of Genetics and Environment
So far, the cause of MSA is unknown. No mutations have been identified in the coding region of SNCA [47]. COQ2 mutations, linked to mitochondrial dysfunction, have been linked to family cases of MSA in the Japanese population, but not in other cohorts [48, 49]. Genome wide association studies (GWAS) identified several potentially interesting gene loci, including the FBXO47, ELOVL7, EDN1, and MAPT, but no association of SNCA and COQ2 variants with MSA [50]. Importantly, MSA and inflammatory bowel disease have been reported to share common genetics including common variants of the C7 gene supporting immune dysfunction in both disorders [51]. The current understanding is that certain genetic background may predispose to MSA. On the other hand, environmental factors associated with oxidative stress and toxicity like those linked to occupational history of farming may be more common in MSA cases as in controls [52].
In summary, MSA is a multifactorial disorder with a rapid progression and selective neuronal loss possibly mediated by a-syn pathology, oligodendroglial dysfunction, and neuroinflammatory signaling. Disease models are instrumental in understanding the contribution of each of these components in the pathogenesis of the disease and provide a testbed for novel therapeutic approaches for MSA.
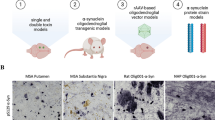
Classes of MSA Models and Their Relevance
Modelling MSA has been approached both in vitro in cell culture and in vivo in rodents and non-human primates. The early in vitro models have been mostly based on a-syn overexpression in glial cells (primary or cell lines) to study the effects of a-syn on their biology in respect to survival [53], susceptibility to oxidative stress and pro-inflammatory signals [54,55,56], and the role of p25a/TPPP in GCI formation [57]. Such models have been further relevant as biosensor systems to study the seeding properties of MSA-derived a-syn oligomers versus those derived from PD brains [58]. Recently, induced pluripotent stem cells (iPSCs) have been reprogrammed from somatic cells (fibroblasts or blood cells) of MSA patients and further differentiated into neurons disclosing possible mitochondrial dysfunction in MSA as compared to cells of healthy controls [59, 60]. MSA iPSC-derived neural progenitor cells (NPCs), which give rise to both neuronal and glial cells, have been found to compensate functionally the putative mitochondrial deficit at baseline as compared to cells of healthy controls. However, the MSA cellular pathology becomes apparent in the dish after exposure to very low doses of oxidative stress [61], further consolidating the idea of the multifactorial origin of MSA with a combined role of a genetic predisposition and environmental trigger.
The in vivo models of MSA have been focusing on replicating the neuropathological and symptomatic phenotype of the disease. Initial neurotoxin models tried to replicate the SND by combining selective striatal and nigral neurotoxins [62,63,64,65,66,67]. These models have been instrumental to study the pathophysiology of SND, but their major limitation has been the lack of a-syn pathology.
Recently, the finding of MSA-specific a-syn strains [30, 31, 58] and their prion-like spreading [68,69,70] has triggered not only significant interest in this rare disease, but has opened a new avenue for preclinical in vivo modelling based on the strain-specific spreading of a-syn [71]. However, typical GCIs, which are widely spread in the human MSA brain, are not readily seen in the rodent brain after intracerebral inoculation of a-syn fibrils. This discrepancy may be due to the different neurobiology of mice and humans, the limited experimental observation periods, a difference between the in vitro generated PFFs and the pathological a-syn fibrils in patients, or other technical issues. Similar observations have been seen when a-syn fibrils have been introduced through the external urethral sphincter or detrusor, propagating to the CNS [72]. The general notion from an increasing number of studies using a-syn spreading models confirms that healthy oligodendrocytes are not readily accumulating fibrillar a-syn in their cytoplasm and possibly a preceding oligodendroglial dysfunction is needed to trigger the GCI formation. This has been supported by a recent experiment, in which only transgenic mice with oligodendroglial a-syn overexpression which get intracerebral inoculation of a-syn polymorphs are prone to accelerating an MSA-like phenotype in a strain-dependent manner [73]. The a-syn spreading models are crucial for understanding the specific features of protein misfolding and properties in MSA versus other synucleinopathies, and thus shed light on pathogenic mechanisms involved in the progression of the disease [74]. Unfortunately, to date, these models have not been able to recapitulate convincingly MSA symptomatology with its characteristic underlying selective neurodegenerative pathology.
The third strategy to model MSA has been the overexpression of a-syn in oligodendrocytes either in constitutive or inducible transgenic mice [75,76,77,78,79] or by AAV targeted a-syn overexpression in the substantia nigra and striatum of mice, rats, or primates [80,81,82,83]. In all overexpression models, irrespective of the mode of overexpression, a delayed progressive neurodegeneration with variable phenotype and intensity has been identified to accompany the formation of GCI-like structures in parallel to signs of neuroinflammation. All these findings have supported the causative role of oligodendroglial a-synucleinopathy in MSA neurodegeneration. However, related to the specific overexpression approach, different patterns of selective neurodegeneration, neuroinflammation, and specific functional phenotypes have been reported.
The PLP-a-Syn Transgenic Mouse — Progressive MSA-Like Neuropathology, Motor, and Non-motor Phenotype
The PLP-a-syn transgenic mouse is generated by overexpression of human wild-type a-syn under the proteolipid protein (PLP) promoter to drive the transgene in oligodendrocytes [75]. This genetic modification results in the progressive accumulation, oligomerization, and aggregation of a-syn in oligodendrocytes throughout the CNS replicating the GCI pathology of human MSA [75, 78]. This finding is similar to observations in other transgenic mice with constitutive overexpression of human a-syn in oligodendroglia under alternative cell-specific promoters like the myelin basic protein (MBP) or the 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNP) promoters [76, 77]. Intriguingly, the PLP-a-syn mouse shows progressive nigral and striatal neurodegeneration, modelling SND of the MSA-P subtype [78]. Nigral neuronal loss is detectable already at 4 months of age of the PLP-a-syn mice [18], while the loss of GABAergic medium spiny neurons in the striatum is detected at 12 months of age [78]. In comparison, the MBP-a-syn mouse model has been recently suggested to represent a model of MSA-C with loss of Purkinje cells detected at 4 months of age [84]. Interestingly, the PLP-a-syn mouse shows increased vulnerability of the olivopontocerebellar system to exogenous mitochondrial stress induced by 3-nitropropionic acid [85] and proteolytic dysfunction triggered by proteasome inhibition [86], leading to OPCA in this MSA model, but never in wild-type mice. Linked to this underlying neuropathology, the PLP-a-syn mouse shows progressive motor disability becoming overt at 6 months of age including shortened stride length, elevated stride length variability, slowness, and loss of balance and coordination evidenced in beam walking and, later on, in pole climbing [78]. In addition, the PLP-a-syn mouse model presents several non-motor deficits, which replicate classical non-motor symptoms in MSA. Among those is the neurogenic bladder dysfunction with a typical detrusor-sphincter dyssynergia and increased postvoid residual urine volume, associated with early loss of parasympathetic outflow neurons in the lumbosacral intermediate columns of the spinal cord already at 2 months of age and delayed degeneration of the pontine micturition center [87]. Brainstem centers, including the locus coeruleus, the nucleus ambiguus, the laterodorsal tegmental nucleus, and the pedunculopontine tegmental nucleus, degenerate early in the individual life of PLP-a-syn mice [88, 89]. This pathology leads to cardiovascular symptoms (increased heart rate variability [88]), respiratory deficits [90], and sleep disturbances including rapid eye movement (REM) sleep without atonia [91] replicating human MSA premotor symptoms [9]. Intriguingly, the widespread GCI pathology in the PLP-a-syn mouse brain leads to strictly selective neuronal loss. For example, the olfactory bulbs show oligodendroglial a-syn accumulation without loss of tyrosine hydroxylase-positive neurons and no disturbances in the olfactory function [92], which recapitulates human MSA in contrast to the early loss of smell in PD [93, 94].
In summary, the PLP-a-syn mouse provides an excellent phenotypic replication of human MSA that proves the strong face validity of the model (Fig. 1, Fig. 2). This MSA mouse has served to study the mechanisms of MSA neurodegeneration and understand the causative role of oligodendroglial a-synucleinopathy. It recapitulates oligodendroglial a-syn-triggered SND with increased vulnerability of the olivopontocerebellar system to exogenous stressors as well as degeneration in autonomic centers. In addition, the PLP-a-syn mouse presents with progressive region-specific microglial activation triggered by the oligomeric a-syn accumulation [78] reminiscent of the microgliosis reported in postmortem analysis of MSA brains [14, 15]. The analysis of the model proposes important role of microglial activation and neuroinflammatory responses in driving the progression of the disease [18, 78]. Alternatively, demyelination is seen in end-stage MSA [15], while in the PLP-a-syn mouse, myelin dysfunction is detected without evident loss of myelin up to 18 months of age [78, 95]. Similarly, astrogliosis is not a prominent pathological feature in the progression of disease in the PLP-a-syn mouse model [78], despite the presence of astrogliosis in the postmortem MSA brains [96]. Prominent demyelination and astrogliosis may represent secondary late events in the pathogenesis of MSA and due to the limited observation time and overall duration of the disease in the PLP-a-syn model may not be detected in the mouse brain. However, demyelination and astrogliosis, but not microglial activation and SND, are reported as part of the neurodegenerative process in the MBP-a-syn mouse [76, 97], proposing that oligodendroglial a-syn overexpression in the two transgenic models may switch on different pathogenic pathways, defining different selective neurodegeneration and phenotype. This difference has not been addressed to date, but one putative explanation may be the generation of different a-syn oligomeric polymorphs, which define each of the phenotypes.
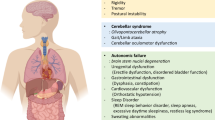
The PLP-a-syn mouse model of MSA — an excellent phenotypic replication of human MSA. Both premotor and progressive motor symptoms are replicated in the PLP-a-syn mouse comparable to the human clinical presentation (green overlaps). The limitations of the model are related to: (i) the initiation of the disease — elusive in human MSA versus a-syn overexpression in oligodendrocytes in PLP-a-syn mice; (ii) the milder phenotype and usually normal lifespan in PLP-a-syn mice versus severe disability and premature death of MSA patients; and (iii) significant differences in the normal biology between mice and humans (lifespan measured in months versus years, respectively), which may interfere with time of disease progression. Created with BioRender.com
The neuropathology in PLP-a-syn mice — recapitulation of the postmortem findings in human MSA and definition of major therapeutic targets. Legend: (1) glial cytoplasmic inclusions (GCIs) are the hallmark of human MSA pathology with unknown origin, while in transgenic mice, GCIs are triggered by overexpression of human a-syn under the PLP promotor in oligodendroglia, which over time leads to high molecular weight a-syn aggregates [78]; neuronal a-syn in vesicular bodies has been observed in dopaminergic nigral neurons possibly due to cell-to-cell propagation of the oligomeric forms [78]; (3) multiple autonomic centers in the brain and spinal cord of PLP-a-syn mice show selective neuronal loss [87,88,89,90]; (4) nigral and striatal neuronal loss feature the underlying motor pathology in PLP-a-syn mice [78]; (5) olivopontocerebellar pathology can be triggered by mitochondrial or proteolytic stress in the PLP-a-syn mouse [85, 86]; (6–8) the neurodegeneration is mediated through gliosis and neuroinflammatory signaling [18, 85, 112]; (9) early signs of oligodendroglial and myelin dysfunction [95] are accelerated by proteolytic stress [86]; and (10) iron deposition in the degenerating areas can be identified [102, 105]. Created with BioRender.com
The MSA Transgenic Mouse — a Preclinical Therapeutic Testbed for MSA with Advantages and Limitations
Preclinical therapeutic screening provides the rationale for any clinical trial. The relevance of the applied experimental model to the tested therapeutic target is crucial to ensure meaningful outcomes. All relevant current MSA models are based on the assumption that pathological a-syn is the cause of MSA neurodegeneration. On one hand, such a-syn models of MSA are advantageous when testing a-syn targeting treatment strategies, because they provide a clear-cut readout of efficacy based on a-syn pathology modulation [98,99,100,101,102,103,104,105]. Furthermore, the a-syn-based models of MSA provide the possibility to screen targeting of other relevant pathways and disease mechanisms downstream of a-syn pathology like neuroinflammation [18, 19, 78, 106, 107], neurotrophic disbalance [108, 109], epigenetic impairment [110], or demyelination [97]. On the other hand, the mechanistic replication of a-synucleinopathy in the rodent CNS may deviate from the actual trigger(s) of the human disease, as the etiology of the disease remains elusive. In combination, the limited knowledge on the initiation of MSA, the inter-species neurobiological differences between rodents and humans, and the common deviation in study design (drug dose, time of therapy initiation and relative duration, readouts, etc.) between preclinical studies and clinical trials may contribute in part to the still disappointing outcomes of clinical trials in MSA. The lack of relevant biomarkers, which may serve to monitor the biological activity of any intervention in relation to slowing disease progression in MSA patients, has been critical. The recent reports on possible progression biomarkers like neurofilament light chain [111] or neuroimaging features including advances in a-syn imaging will be crucial to provide relevant measures of target engagement in the near future.
Conclusions and Future Directions
In summary, the models with targeted oligodendroglial overexpression of wild-type human a-syn provide a good replication of MSA-like neurodegeneration induced by oligodendroglial a-synucleinopathy. The PLP-a-syn mouse offers the most complete mechanistic recapitulation of the MSA phenotype with neurogenic bladder dysfunction, cardiovascular and respiratory deficits, REM sleep abnormalities, and progressive motor disability with underlying striatonigral degeneration and increased susceptibility of the olivopontocerebellar system to exogenous stress factors like oxidative or proteolytic stress. The limitations of the model relate to the differences in the neurobiology between mice and humans as well as the lacking information on the initiation event(s) in human MSA that makes their replication obscure in any of the current models. The PLP-a-syn mouse serves well to test the target engagement and efficacy of therapies targeting a-syn pathology and downstream pathways including neuroinflammation, disrupted neurotrophic support, and others (Fig. 2); however, its positive predictive validity has not yet been confirmed. Future development of MSA models involving human disease-specific cells may be needed to overcome the existing limitations and provide better preclinical tools for biomarker and treatment development for MSA.
Required Author Form
Disclosure form provided by the author are available with the online version of this article.
References
Graham JG, Oppenheimer DR. Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1969;32:28–34.
Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J Neurol Sci. 1989;94:79–100.
Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–8.
Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, et al. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol (Berl). 1998;96:445–52.
Gai WP, Power JH, Blumbergs PC, Blessing WW. Multiple-system atrophy: a new alpha-synuclein disease? Lancet. 1998;352:547–8.
Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology. 1997;49:1284–8.
Schrag A, Ben Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–5.
O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain. 2008;131:1362–72.
Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. 2015;372:249–63.
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6.
Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85:404–12.
Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204.
Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995;58:160–6.
Ishizawa K, Komori T, Sasaki S, Arai N, Mizutani T, Hirose T. Microglial activation parallels system degeneration in multiple system atrophy. J Neuropathol Exp Neurol. 2004;63:43–52.
Ishizawa K, Komori T, Arai N, Mizutani T, Hirose T. Glial cytoplasmic inclusions and tissue injury in multiple system atrophy: a quantitative study in white matter (olivopontocerebellar system) and gray matter (nigrostriatal system). Neuropathology. 2008;28:249–57.
Matsuo A, Akiguchi I, Lee GC, McGeer EG, McGeer PL, Kimura J. Myelin degeneration in multiple system atrophy detected by unique antibodies. Am J Pathol. 1998;153:735–44.
Song YJ, Lundvig DM, Huang Y, Gai WP, Blumbergs PC, Hojrup P, et al. p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol. 2007;171:1291–303.
Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord. 2007;22:2196–203.
Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21:393–404.
Li F, Ayaki T, Maki T, Sawamoto N, Takahashi R. NLRP3 inflammasome-related proteins are upregulated in the putamen of patients with multiple system atrophy. J Neuropathol Exp Neurol. 2018;77:1055–65.
Starhof C, Winge K, Heegaard NHH, Skogstrand K, Friis S, Hejl A. Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes. J Neuroinflammation. 2018;15:305.
Refolo V, Stefanova N. Neuroinflammation and glial phenotypic changes in alpha-synucleinopathies. Front Cell Neurosci. 2019;13:263.
Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64:239–46.
Nishie M, Mori F, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, et al. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol. 2004;107:292–8.
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–9.
Arima K, Murayama S, Mukoyama M, Inose T. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 1. Neuronal cytoplasmic inclusions. Acta Neuropathol. 1992;83:453–60.
Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–45.
Lin WL, DeLucia MW, Dickson DW. Alpha-synuclein immunoreactivity in neuronal nuclear inclusions and neurites in multiple system atrophy. Neurosci Lett. 2004;354:99–102.
Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, et al. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–309.
Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X, et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–7.
Schweighauser M, Shi Y, Tarutani A, Kametani F, Murzin AG, Ghetti B, et al. Structures of α-synuclein filaments from multiple system atrophy. Nature. 2020.
Jellinger KA, Lantos PL. Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol. 2010;119:657–67.
Bukhatwa S, Zeng BY, Rose S, Jenner P. A comparison of changes in proteasomal subunit expression in the substantia nigra in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Brain Res. 2010;1326:174–83.
Masui K, Nakata Y, Fujii N, Iwaki T. Extensive distribution of glial cytoplasmic inclusions in an autopsied case of multiple system atrophy with a prolonged 18-year clinical course. Neuropathology. 2012;32:69–76.
Miki Y, Tanji K, Mori F, Tatara Y, Utsumi J, Sasaki H, et al. AMBRA1, a novel alpha-synuclein-binding protein, is implicated in the pathogenesis of multiple system atrophy. Brain Pathol. 2016.
Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm. 2005;112:1613–24.
Asi YT, Simpson JE, Heath PR, Wharton SB, Lees AJ, Revesz T, et al. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia. 2014;62:964–70.
Djelloul M, Holmqvist S, Boza-Serrano A, Azevedo C, Yeung MS, Goldwurm S, et al. Alpha-synuclein expression in the oligodendrocyte lineage: an in vitro and in vivo study using rodent and human models. Stem Cell Reports. 2015;5:174–84.
May VE, Ettle B, Poehler AM, Nuber S, Ubhi K, Rockenstein E, et al. Alpha-synuclein impairs oligodendrocyte progenitor maturation in multiple system atrophy. Neurobiol Aging. 2014;35:2357–68.
Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL. Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol. 2013;23:263–73.
Wakabayashi K, Mori F, Nishie M, Oyama Y, Kurihara A, Yoshimoto M, et al. An autopsy case of early (“minimal change”) olivopontocerebellar atrophy (multiple system atrophy-cerebellar). Acta Neuropathol. 2005;110:185–90.
Ling H, Asi YT, Petrovic IN, Ahmed Z, Prashanth LK, Hazrati LN, et al. Minimal change multiple system atrophy: an aggressive variant? Mov Disord. 2015;30:960–7.
Wenning GK, Ben Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinical features and natural history of multiple system atrophy. An analysis of 100 cases. Brain. 1994;117 ( Pt 4):835–45.
Vacchi E, Senese C, Chiaro G, Disanto G, Pinton S, Morandi S, et al. Alpha-synuclein oligomers and small nerve fiber pathology in skin are potential biomarkers of Parkinson’s disease. NPJ Parkinson’s disease. 2021;7:119.
Nakamura K, Mori F, Kon T, Tanji K, Miki Y, Tomiyama M, et al. Filamentous aggregations of phosphorylated alpha-synuclein in Schwann cells (Schwann cell cytoplasmic inclusions) in multiple system atrophy. Acta Neuropathol Commun. 2015;3:29.
Ozawa T, Shimizu H, Matsui H, Onodera O, Kakita A. Shrinkage of the myenteric neurons of the small intestine in patients with multiple system atrophy. Autonomic neuroscience: basic & clinical. 2019;221: 102583.
Ozawa T, Takano H, Onodera O, Kobayashi H, Ikeuchi T, Koide R, et al. No mutation in the entire coding region of the alpha-synuclein gene in pathologically confirmed cases of multiple system atrophy. Neurosci Lett. 1999;270:110–2.
Ogaki K, Fujioka S, Heckman MG, Rayaprolu S, Soto-Ortolaza AI, Labbe C, et al. Analysis of COQ2 gene in multiple system atrophy. Mol Neurodegener. 2014;9:44.
Collaboration. TM-SAR. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233–44.
Sailer A, Scholz SW, Nalls MA, Schulte C, Federoff M, Price TR, et al. A genome-wide association study in multiple system atrophy. Neurology. 2016;87:1591–8.
Shadrin AA, Mucha S, Ellinghaus D, Makarious MB, Blauwendraat C, Sreelatha AAK, et al. Shared genetics of multiple system atrophy and inflammatory bowel disease. Mov Disord. 2021;36:449–59.
Vanacore N, Bonifati V, Fabbrini G, Colosimo C, De Michele G, Marconi R, et al. Case-control study of multiple system atrophy. Mov Disord. 2005;20:158–63.
Tsuboi K, Grzesiak JJ, Bouvet M, Hashimoto M, Masliah E, Shults CW. Alpha-synuclein overexpression in oligodendrocytic cells results in impaired adhesion to fibronectin and cell death. Mol Cell Neurosci. 2005;29:259–68.
Stefanova N, Klimaschewski L, Poewe W, Wenning GK, Reindl M. Glial cell death induced by overexpression of alpha-synuclein. J Neurosci Res. 2001;65:432–8.
Stefanova N, Emgard M, Klimaschewski L, Wenning GK, Reindl M. Ultrastructure of alpha-synuclein-positive aggregations in U373 astrocytoma and rat primary glial cells. Neurosci Lett. 2002;323:37–40.
Stefanova N, Schanda K, Klimaschewski L, Poewe W, Wenning GK, Reindl M. Tumor necrosis factor-alpha-induced cell death in U373 cells overexpressing alpha-synuclein. J Neurosci Res. 2003;73:334–40.
Mavroeidi P, Arvanitaki F, Karakitsou AK, Vetsi M, Kloukina I, Zweckstetter M, et al. Endogenous oligodendroglial alpha-synuclein and TPPP/p25α orchestrate alpha-synuclein pathology in experimental multiple system atrophy models. Acta Neuropathol. 2019;138:415–41.
Yamasaki TR, Holmes BB, Furman JL, Dhavale DD, Su BW, Song ES, et al. Parkinson’s disease and multiple system atrophy have distinct α-synuclein seed characteristics. J Biol Chem. 2019;294:1045–58.
Monzio Compagnoni G, Kleiner G, Samarani M, Aureli M, Faustini G, Bellucci A, et al. Mitochondrial dysregulation and impaired autophagy in iPSC-derived dopaminergic neurons of multiple system atrophy. Stem Cell Reports. 2018;11:1185–98.
Nakamoto FK, Okamoto S, Mitsui J, Sone T, Ishikawa M, Yamamoto Y, et al. The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci Rep. 2018;8:14215.
Herrera-Vaquero M, Heras-Garvin A, Krismer F, Deleanu R, Boesch S, Wenning GK, et al. Signs of early cellular dysfunction in multiple system atrophy. Neuropathol Appl Neurobiol. 2021;47:268–82.
Wenning GK, Granata R, Laboyrie PM, Quinn NP, Jenner P, Marsden CD. Reversal of behavioural abnormalities by fetal allografts in a novel rat model of striatonigral degeneration. Mov Disord. 1996;11:522–32.
Scherfler C, Puschban Z, Ghorayeb I, Goebel GP, Tison F, Jellinger K, et al. Complex motor disturbances in a sequential double lesion rat model of striatonigral degeneration (multiple system atrophy). Neuroscience. 2000;99:43–54.
Ghorayeb I, Fernagut PO, Aubert I, Bezard E, Poewe W, Wenning GK, et al. Toward a primate model of L-dopa-unresponsive parkinsonism mimicking striatonigral degeneration. Mov Disord. 2000;15:531–6.
Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, et al. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000;95:377–88.
Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, et al. Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57B1/6 mice: behavioural and histopathological characterisation. Neuroscience. 2002;114:1005–17.
Stefanova N, Puschban Z, Fernagut PO, Brouillet E, Tison F, Reindl M, et al. Neuropathological and behavioral changes induced by various treatment paradigms with MPTP and 3-nitropropionic acid in mice: towards a model of striatonigral degeneration (multiple system atrophy). Acta Neuropathol (Berl). 2003;106:157–66.
Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015.
Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, et al. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci U S A. 2013;110:19555–60.
Jellinger KA, Wenning GK, Stefanova N. Is multiple system atrophy a prion-like disorder? Int J Mol Sci. 2021;22.
Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, et al. Alpha-synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–4.
Ding X, Zhou L, Jiang X, Liu H, Yao J, Zhang R, et al. Propagation of pathological α-synuclein from the urogenital tract to the brain initiates MSA-like syndrome. iScience. 2020;23:101166.
Torre-Muruzabal T, Van der Perren A, Coens A, Gelders G, Barber Janer A, Camacho-Garcia S, et al. Host oligodendrogliopathy and ɑ-synuclein strains dictate disease severity in multiple system atrophy. Brain. 2022.
Woerman AL, Oehler A, Kazmi SA, Lee J, Halliday GM, Middleton LT, et al. Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Acta Neuropathol. 2019;137:437–54.
Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Spooren W, et al. Hyperphosphorylation and insolubility of alpha-synuclein in transgenic mouse oligodendrocytes. EMBO Rep. 2002;3:583–8.
Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, et al. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005;25:10689–99.
Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–59.
Refolo V, Bez F, Polissidis A, Kuzdas-Wood D, Sturm E, Kamaratou M, et al. Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies. Acta Neuropathol Commun. 2018;6:2.
Tanji K, Miki Y, Mori F, Nikaido Y, Narita H, Kakita A, et al. A mouse model of adult-onset multiple system atrophy. Neurobiol Dis. 2019;127:339–49.
Mandel RJ, Marmion DJ, Kirik D, Chu Y, Heindel C, McCown T, et al. Novel oligodendroglial alpha synuclein viral vector models of multiple system atrophy: studies in rodents and nonhuman primates. Acta Neuropathol Commun. 2017;5:47.
Williams GP, Marmion DJ, Schonhoff AM, Jurkuvenaite A, Won WJ, Standaert DG, et al. T cell infiltration in both human multiple system atrophy and a novel mouse model of the disease. Acta Neuropathol. 2020;139:855–74.
Marmion DJ, Rutkowski AA, Chatterjee D, Hiller BM, Werner MH, Bezard E, et al. Viral-based rodent and nonhuman primate models of multiple system atrophy: fidelity to the human disease. Neurobiol Dis. 2021;148: 105184.
Bassil F, Guerin PA, Dutheil N, Li Q, Klugmann M, Meissner WG, et al. Viral-mediated oligodendroglial alpha-synuclein expression models multiple system atrophy. Mov Disord. 2017;32:1230–9.
Mészáros L, Riemenschneider MJ, Gassner H, Marxreiter F, von Hörsten S, Hoffmann A, et al. Human alpha-synuclein overexpressing MBP29 mice mimic functional and structural hallmarks of the cerebellar subtype of multiple system atrophy. Acta Neuropathol Commun. 2021;9:68.
Stefanova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, et al. Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol. 2005;166:869–76.
Stefanova N, Kaufmann WA, Humpel C, Poewe W, Wenning GK. Systemic proteasome inhibition triggers neurodegeneration in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. Acta Neuropathol. 2012;124:51–65.
Boudes M, Uvin P, Pinto S, Voets T, Fowler CJ, Wenning GK, et al. Bladder dysfunction in a transgenic mouse model of multiple system atrophy. Mov Disord. 2013;28:347–55.
Kuzdas D, Stemberger S, Gaburro S, Stefanova N, Singewald N, Wenning GK. Oligodendroglial alpha-synucleinopathy and MSA-like cardiovascular autonomic failure: experimental evidence. Exp Neurol. 2013;247:531–6.
Stemberger S, Poewe W, Wenning GK, Stefanova N. Targeted overexpression of human alpha-synuclein in oligodendroglia induces lesions linked to MSA-like progressive autonomic failure. Exp Neurol. 2010;224:459–64.
Flabeau O, Meissner WG, Ozier A, Berger P, Tison F, Fernagut PO. Breathing variability and brainstem serotonergic loss in a genetic model of multiple system atrophy. Mov Disord. 2014;29:388–95.
Hartner L, Keil TW, Kreuzer M, Fritz EM, Wenning GK, Stefanova N, et al. Distinct parameters in the EEG of the PLP alpha-SYN mouse model for multiple system atrophy reinforce face validity. Front Behav Neurosci. 2016;10:252.
Krismer F, Wenning GK, Li Y, Poewe W, Stefanova N. Intact olfaction in a mouse model of multiple system atrophy. PLoS ONE. 2013;8: e64625.
Postuma RB, Pelletier A, Gagnon JF, Montplaisir J. Evolution of prodromal multiple system atrophy from REM sleep behavior disorder: a descriptive study. Journal of Parkinson’s disease. 2022;12:983–91.
Moscovich M, Heinzel S, Postuma RB, Reilmann R, Klockgether T, Jacobi H, et al. How specific are non-motor symptoms in the prodrome of Parkinson’s disease compared to other movement disorders? Parkinsonism Relat Disord. 2020;81:213–8.
Schafferer S, Khurana R, Refolo V, Venezia S, Sturm E, Piatti P, et al. Changes in the miRNA-mRNA regulatory network precede motor symptoms in a mouse model of multiple system atrophy: clinical implications. PLoS ONE. 2016;11: e0150705.
Dickson DW. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2.
Ettle B, Kerman BE, Valera E, Gillmann C, Schlachetzki JC, Reiprich S, et al. Alpha-synuclein-induced myelination deficit defines a novel interventional target for multiple system atrophy. Acta Neuropathol. 2016;132:59–75.
Heras-Garvin A, Weckbecker D, Ryazanov S, Leonov A, Griesinger C, Giese A, et al. Anle138b modulates α-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy. Mov Disord. 2019;34:255–63.
Lemos M, Venezia S, Refolo V, Heras-Garvin A, Schmidhuber S, Giese A, et al. Targeting α-synuclein by PD03 AFFITOPE® and Anle138b rescues neurodegenerative pathology in a model of multiple system atrophy: clinical relevance. Translational neurodegeneration. 2020;9:38.
Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, et al. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10:10.
Herrera-Vaquero M, Bouquio D, Kallab M, Biggs K, Nair G, Ochoa J, et al. The molecular tweezer CLR01 reduces aggregated, pathologic, and seeding-competent alpha-synuclein in experimental multiple system atrophy. Biochim Biophys Acta Mol Basis Dis. 2019.
Heras-Garvin A, Refolo V, Schmidt C, Malfertheiner K, Wenning GK, Bradbury M, et al. ATH434 reduces α-synuclein-related neurodegeneration in a murine model of multiple system atrophy. Mov Disord. 2021.
Bassil F, Fernagut PO, Bezard E, Pruvost A, Leste-Lasserre T, Hoang QQ, et al. Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy. Proc Natl Acad Sci U S A. 2016;113:9593–8.
Ubhi K, Rockenstein E, Mante M, Patrick C, Adame A, Thukral M, et al. Rifampicin reduces alpha-synuclein in a transgenic mouse model of multiple system atrophy. NeuroReport. 2008;19:1271–6.
Shukla JJ, Stefanova N, Bush AI, McColl G, Finkelstein DI, McAllum EJ. Therapeutic potential of iron modulating drugs in a mouse model of multiple system atrophy. Neurobiol Dis. 2021;159: 105509.
Kaindlstorfer C, Sommer P, Georgievska B, Mather RJ, Kugler AR, Poewe W, et al. Failure of neuroprotection despite microglial suppression by delayed-start myeloperoxidase inhibition in a model of advanced multiple system atrophy: clinical implications. Neurotox Res. 2015;28:185–94.
Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal alpha-synucleinopathy. Mol Neurodegener. 2017;12:52.
Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;30:6236–46.
Bassil F, Canron MH, Vital A, Bezard E, Li Y, Greig NH, et al. Insulin resistance and exendin-4 treatment for multiple system atrophy. Brain. 2017.
Sturm E, Fellner L, Krismer F, Poewe W, Wenning GK, Stefanova N. Neuroprotection by epigenetic modulation in a transgenic model of multiple system atrophy. Neurotherapeutics. 2016;13:871–9.
Zhang L, Cao B, Hou Y, Gu X, Wei Q, Ou R, et al. Neurofilament light chain predicts disease severity and progression in multiple system atrophy. Mov Disord. 2022;37:421–6.
Stemberger S, Jamnig A, Stefanova N, Lepperdinger G, Reindl M, Wenning GK. Mesenchymal stem cells in a transgenic mouse model of multiple system atrophy: immunomodulation and neuroprotection. PLoS ONE. 2011;6: e19808.
Funding
Open access funding provided by Austrian Science Fund (FWF). This work has been supported by grants of the Austrian Science Fund (FWF) F4414 and W1206-08.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stefanova, N. A Mouse Model of Multiple System Atrophy: Bench to Bedside. Neurotherapeutics 20, 117–126 (2023). https://doi.org/10.1007/s13311-022-01287-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01287-8