Abstract
Hyperglycemia is associated with decreased recanalization probability and increased risk of hemorrhagic complications for stroke patients treated with intravenous alteplase. However, whether hyperglycemia modifies alteplase treatment effect on clinical outcome in patients with large vessel occlusion stroke undergoing endovascular thrombectomy is uncertain. We conducted this study to determine a possible interaction effect between admission hyperglycemia and intravenous alteplase prior to thrombectomy in patients with large vessel occlusion stroke. In this post-hoc analysis of a randomized trial (DIRECT-MT) comparing intravenous alteplase before endovascular treatment vs. endovascular treatment only, 649 with available baseline glucose measurements were included. The treatment-by-admission hyperglycemia (defined as plasma glucose levels ≥ 7.8 mmol/L [140 mg/dL]) interaction was assessed using logistic regression models. As a result, among 649 patients included, 224 (34.5%) were hyperglycemic at admission. There was evidence of alteplase treatment effect modification by hyperglycemia (Pinteraction = 0.025). In patients without hyperglycemia, combination therapy was associated with better outcomes compared to mechanical thrombectomy alone (adjusted common odd ratio [acOR] 1.46, 95% CI [1.04–2.07]), but not in hyperglycemic patients (acOR 0.74, 95% CI [0.46–1.20]). Combination therapy led to an absolute increase of 6% excellent outcome (mRS 0–1) in non-hyperglycemic patients (aOR 1.71, 95% CI [1.05–2.79]), but resulted in a 12.3% absolute decrease (aOR 0.42 [95% CI, 0.19–0.95] in hyperglycemic patients (Pinteraction = 0.003). In conclusion, for large vessel occlusion patients directly presenting to a thrombectomy-capable hospital, hyperglycemia modified combination treatment effect on clinical outcome. Combination therapy was beneficial in patients without hyperglycemia, while thrombectomy alone may be preferred in hyperglycemic patients. Further studies are needed to confirm this result.
Trial Registration Information: clinicaltrials.gov Identifier: NCT03469206.
Similar content being viewed by others
Introduction
Hyperglycemia at admission is a common phenomenon in acute ischemic stroke, occurring in 30–50% of patients [1, 2]. Various mechanisms in which hyperglycemia accelerates ischemic tissue damage in acute ischemic stroke have been described, including impaired reactivity of the cerebral microvasculature, alterations in blood–brain barrier permeability, cortical acidosis, production of reactive oxygen and nitrogen species, and hypercoagulability which may finally increase infarct size and lead to brain swelling and hemorrhagic transformation [3,4,5]. Intravenous alteplase is a tissue fibrinogen activator that dissolves intracranial thrombi. Observational studies in large vessel occlusion stroke patients treated with intravenous alteplase showed that hyperglycemia may hamper the fibrinolytic process, which not only decreases the probability of recanalization [6, 7], but also increases the risk of symptomatic intracerebral hemorrhage [7, 8], thereby ultimately leading to poorer outcomes, both with and without concurrent endovascular thrombectomy.
Currently, combined treatment with intravenous alteplase and endovascular thrombectomy (EVT) constitutes the standard of care for patients with large vessel occlusion stroke [9, 10]. However, the added value of intravenous alteplase in patients with large vessel occlusion stroke who present directly to an thrombectomy-capable hospital is controversial. Five recent randomized clinical trials (DIRECT-MT, SKIP, DEVT and MR CLEAN-NO IV, SWIFT-DIRECT) have investigated this question, with different results: while the DIRECT-MT and DEVT trials showed non-inferiority of EVT alone compared to EVT with concurrent intravenous alteplase, SKIP, MR CLEAN-NO IV, and SWIFT-DIRECT did not [11,12,13,14,15]. Although more evidence is necessary, these studies show that EVT alone may achieve functional outcomes comparable to a combination therapy of EVT and intravenous alteplase. Given these observations, and in light of the decreased recanalization probability and increased risk of hemorrhagic complications with intravenous alteplase in the setting of hyperglycemia reported in the literatures [1, 4, 6, 16, 17], it may be beneficial to forego alteplase treatment in patients with hyperglycemia. However, there are no convincing clinical data supporting this assumption yet.
DIRECT-MT was a randomized controlled trial evaluating thrombectomy alone versus combination therapy (thrombectomy preceded by intravenous alteplase) for acute ischemic stroke patients with large vessel occlusion in the anterior circulation, who presented directly to an EVT-capable center [11]. In this post-hoc analysis, we investigated whether admission hyperglycemia modifies IV alteplase treatment effect on clinical outcome.
Methods
The raw data underlying this analysis will be made available by the corresponding author upon reasonable request and after approval by the DIRECT-MT investigators and Human Genetic Resource Administration of China.
Study Design and Patients
DIRECT-MT was a randomized, controlled, open-label trial, assessing non-inferiority of EVT endovascular thrombectomy alone versus combination therapy (thrombectomy proceeded by intravenous alteplase) in patients from 41 academic tertiary care centers in China presenting directly to an endovascular treatment capable center. Detailed inclusion and exclusion criteria and trial design of the DIRECT-MT trial have been reported elsewhere [11, 18]. Patients meeting the trial eligibility criteria were randomly assigned in a 1:1 ratio to undergo endovascular thrombectomy alone or endovascular thrombectomy preceded by intravenous alteplase, at a dose of 0.9 mg per kilogram of body weight, administered within 4.5 h after symptom onset.
All patients or their legal representatives provided written informed consent before randomization. The study protocol was approved by a central medical ethics committee and the research board of each participating center. In this post-hoc analysis, we included patients in whom admission glucose plasma levels were captured. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Clinical Data Collection
Patient demographic and clinical characteristics, including a comprehensive blood workup, were recorded at the time of enrollment. Patient plasma glucose was measured at baseline in venous blood samples, immediately upon hospital admission using GOD (glucose oxidase)-POD (peroxidase) method. Based on common convention, hyperglycemia was defined as plasma blood glucose ≥ 7.8 mmol/L (140 mg/dL) [19, 20]. Clinical evaluation was repeated during the course of the hospital, with additional clinical assessments at 24 h and 5–7 days or discharge.
Imaging Data Evaluation
All patients underwent both non-contrast computed tomography (NCCT) of the head and CTA of the head and neck vessels at baseline as standard care. Imaging follow-up with NCCT and CTA was performed at 24–72 h, with an additional NCCT at 5–7 days. Reperfusion quality before and after EVT was assessed on initial and final intracranial catheter angiogram runs using the expanded Thrombolysis in Cerebral Infarction (eTICI) score (ranging from 0 [no reperfusion] to 3 [complete reperfusion]) [21].
All imaging, including catheter angiograms, were evaluated by an independent imaging core laboratory by trained staff who were unaware of the patients’ treatment assignments and baseline characteristics. All imaging was read by two experienced readers, with a consensus reading in case of discrepancies.
Outcomes
The primary outcome was the modified Rankin score (mRS) assessed at 90 days after randomization. The mRS is a 7-point ordinal scale for functional outcome ranging from 0 (no symptoms) to 6 (death) [22], which was assessed via structured interviews that were performed in person or by telephone with the use of standardized forms by local, trained physicians who were unaware of the patients’ trial-group assignments.
Secondary outcomes included excellent clinical outcome (mRS score 0–1 at 90 days); good outcome (mRS score 0–2 at 90 days); and successful reperfusion before and after thrombectomy, defined as an extended Thrombolysis in Cerebral Infarction (eTICI) score of 2b, 2c, or 3 on the first and the last angiogram, respectively [21].
Safety outcomes were mortality at 90 days, evidence of any intracranial hemorrhage during hospitalization, and symptomatic intracranial hemorrhage according to the Heidelberg criteria [23].
Statistical Analysis
All analyses were based on the intention-to-treat population. Baseline variables, primary, secondary, and safety outcomes were presented for patients with vs. without hyperglycemia at baseline, and were compared between groups using χ2 test or Fisher exact test for categorical variables and a t test or Kruskal–Wallis test for continuous variables.
For the primary outcome, unadjusted and adjusted ordinal logistic regression models with ordinal mRS as dependent variable and treatment allocation (combination therapy vs. EVT) as independent variable were used. Adjustment variables were chosen following the methodology from a previous publication from the HERMES collaboration [8], and included age, sex, baseline stroke severity (NIHSS score), occlusion site (intracranial internal carotid artery vs. M1 vs. M2 segment middle cerebral artery occlusion), time from stroke onset to randomization, history of diabetes mellitus (yes/no), and other imbalanced variables across groups (history of hypertension [yes/no], baseline blood pressure). Then, 2-way multiplicative interaction terms (treatment allocation * hyperglycemia) were entered into the models to test the modification of the effect of intravenous alteplase treatment allocation by hyperglycemia.
Similar methods were applied for secondary and safety outcomes. Multiple test adjustment was not planned in this post-hoc exploratory analysis. No imputation was performed for missing data since missing data were minimal. Analyses were performed with the use of SAS software, version 9.4 (SAS Institute). All P values were two sided, with statistical significance at less than 0.05.
The DIRECT-MT trial was registered in clinical trials (Identifier: NCT03469206). Study protocol and statistical analysis plan have been published before and are available in eSAP 1 [11]. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.
Results
Patient Characteristics and Clinical Outcomes in Patients With and Without Hyperglycemia
Between February 23, 2018, and July 2, 2019, 1586 patients were screened for eligibility at 41 sites, and 656 patients were randomized. A detailed flow diagram has been published before [18]. Seven patients without admission glucose information were excluded from this study, leaving 649 patients (325 in the thrombectomy alone group, and 324 in combination therapy group) for the final analysis (Fig. SI).
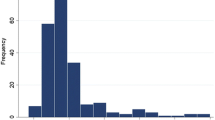
Across the entire study population, the median glucose on admission was 7.0 mmol/L (IQR, 5.9–8.8 mmol/L) (Fig. 1). Two-hundred-twenty-four (34.5%) of the patients were hyperglycemic (≥ 7.8 mmol/L or 140 mg/dL), and 425 (65.3%) were non-hyperglycemic (including 421 normoglycemic [3.9–7.8 mmol/L] and 4 hypoglycemic patients [≤ 3.9 mmol/L or 70 mg/dL]). Baseline characteristics of these patients are summarized in Table 1.
Hyperglycemic patients were older, more often male, had higher baseline systolic blood pressure, and more often had a medical history of diabetes or hypertension, compared to patients without hyperglycemia (Table SI). Patients with hyperglycemia generally suffered poorer outcomes, with a shift towards worse outcomes on ordinal mRS, a lower rate of excellent (mRS score of 0 to 1) and good (mRS score of 0 to 2) outcome, higher mortality, and any higher rates of intracranial hemorrhage. Symptomatic hemorrhage was nominally more frequently observed in the hyperglycemia group, but the difference was not significant (15 [6.7%] versus 19 [4.5%], P = 0.23) (Table SII).
Modification of Treatment Effect by Hyperglycemia
Primary Outcome
There were no significant differences regarding the primary outcome between EVT alone and combination therapy group in the overall study sample (adjusted common ratio [acOR] 1.13, 95% CI [0.85–1.48]; common ratio [cOR] 0.99, 95% CI [0.754–1.291]). No significant treatment-by-hyperglycemia interaction for ordinal mRS was observed in the unadjusted analysis (Pinteraction = 0.055). After adjustment, the differences of treatment effect across groups became however significant (Pinteraction = 0.025), whereby a shift in favor of combination therapy was observed in patients without hyperglycemia (acOR 1.46, 95% CI [1.04–2.07]), while this was not the case in hyperglycemic patients (acOR 0.74, 95% CI [0.46–1.20]) (Table 2, Fig. 2).
Effect of treatment on the distribution of the modified Rankin Scale (mRS) scores at 90 days in non-hyperglycemia and hyperglycemia groups. Numbers in the horizontal bars are percentages. Corresponding adjusted common odds ratios* (acOR) are reported below the bars. *Adjusted for age, sex, baseline stroke severity (NIHSS score), occlusion site (intracranial internal carotid artery/M1/M2), time from stroke onset to randomization, history of diabetes mellitus (yes/no), history of hypertension (yes/no), and baseline blood pressure. Scores range from 0 to 6, with 0 indicating no symptoms, 1 no clinically significant disability, 2 slight disability (patient can function without assistance but cannot carry out all previous activities), 3 moderate disability (patient requires some help but can walk unassisted), 4 moderately severe disability (patient cannot attend to bodily needs without assistance and cannot walk unassisted), 5 severe disability (patient requires constant nursing care and attention), and 6 death
Secondary Outcomes
Similar to the primary outcome, we observed significant treatment-by-hyperglycemia interaction terms for excellent clinical outcome (mRS score of 0 to 1, Pinteraction = 0.003) and good clinical outcome (mRS score of 0 to 2, Pinteraction = 0.048) in adjusted analyses (Table 2). In patients without hyperglycemia, combination therapy led to an absolute increase of 6.0% in excellent outcome (adjusted odd ratio [aOR] 1.71, 95% CI [1.05–2.79]) and 7.4% in good outcome (aOR 1.66, 95% CI [1.06–2.61]). In contrast, combination therapy resulted in 12.3% absolute decrease in excellent outcome (aOR 0.42 [95% CI, 0.19–0.95] and 6.6% absolute decrease in good outcome (aOR 0.78 [95% CI, 0.41–1.49]) (Fig. 2). The treatment-by-hyperglycemia interaction persisted in unadjusted analyses regarding to excellent clinical outcome (unadjusted Pinteraction = 0.005), but not in good clinical outcome (unadjusted Pinteraction = 0.078).
Intravenous alteplase treatment effect on successful reperfusion before and after thrombectomy was modified by hyperglycemia (Pinteraction = 0.014 and 0.008, respectively). Patients without hyperglycemia receiving combination therapy harbored a higher rate of successful reperfusion before (aOR 6.90 [95% CI, 1.99–23.90]) and after thrombectomy (aOR 2.21 [95% CI, 1.27–3.85]), while hyperglycemic patients did not. Interaction remained significant in the unadjusted analyses.
Safety Outcomes
Hyperglycemia modified the alteplase treatment effect on any intracranial hemorrhage (Pinteraction = 0.015), whereby in hyperglycemic patients, combination therapy led to a higher rate of any intracranial hemorrhage (aOR 2.00 [95% CI, 1.14–3.50]). There was no significant interaction regarding symptomatic hemorrhage (Pinteraction = 0.41) and mortality (Pinteraction = 0.46). Results in unadjusted results were similar (Table 2).
Sensitivity Analysis
When treating glucose as a continuous variable, the results were similar. Treatment-by-blood glucose interaction regarding the mRS shift (Pinteraction = 0.006), excellent clinical outcome, pre-DSA or final reperfusion, and any ICH persisted in adjusted analyses, and a shift in favor of thrombectomy alone was observed with increasement of blood glucose. Unadjusted analysis yielded similar results, including the primary outcome (unadjusted Pinteraction = 0.012) (Table SIII, Fig. SII).
Discussion
In this post-hoc analysis of the DIRECT-MT trial, we found that hyperglycemia, defined as admission plasma glucose ≥ 7.8 mmol/L (140 mg/dL), modified the intravenous alteplase treatment effect on clinical outcome. Combination therapy with intravenous alteplase and concurrent endovascular thrombectomy led to improved mRS scores at 90 days, a higher rate of excellent clinical outcome and good outcome only in patients without hyperglycemia, but not in those with hyperglycemia.
Data Interpretation
We observed a significant interaction between treatment allocation (combination therapy vs. EVT) and hyperglycemia on clinical outcome. The difference in alteplase treatment effect between patients with and without hyperglycemia at admission may be partly explained by differences in reperfusion quality (eTICI) and hemorrhage complications.
Administration of intravenous alteplase prior to thrombectomy resulted in a higher reperfusion rate before and after EVT in patients without hyperglycemia, but not in those without hyperglycemia. This result is consistent with a series of previous studies [6, 24, 25], which showed that hyperglycemia inhibits fibrinolysis and thereby hampers the therapeutic effect of intravenous alteplase, ultimately resulting in worse reperfusion quality [6, 7, 26].
We also observed differences in the effect of intravenous alteplase on hemorrhagic complications between patients with and without hyperglycemia on admission. The former harbored a higher rate of any intracranial hemorrhage with intravenous alteplase, while in the latter, the rate of intracranial hemorrhage was similar in patients with and without intravenous alteplase. These findings support previous studies, which demonstrated that hyperglycemia is associated with increased hemorrhage risk following alteplase administration [27,28,29,30].
In summary, hyperglycemia decreased fibrinolytic efficacy of intravenous alteplase and increased the risk of alteplase-related hemorrhagic complications. As a result, there was no clinical benefit of intravenous alteplase treatment in patients with hyperglycemia. On the contrary, an adverse effect on the excellent clinical outcome was observed, perhaps due to the higher hemorrhage rates following alteplase treatment in such patients. Patients without hyperglycemia, on the other hand, benefitted from concurrent alteplase administration both in terms of clinical outcomes and angiographic reperfusion quality.
Comparison with Other Studies
Prior to this study, a series of articles have demonstrated an association of hyperglycemia and poor outcome in acute ischemic stroke patients, but this effect was thought to be independent from any intervention such as intravenous alteplase administration [1, 6, 24, 27, 29].
In this study, we also observed a deleterious effect of hyperglycemia on clinical outcome in acute ischemic stroke patients undergoing endovascular thrombectomy. However, contrary to previous studies, we found that hyperglycemia modifies the alteplase treatment effect on clinical outcome. The discrepancy with previous studies may be caused by the differences in therapeutic interventions. Our data are from the DIRECT-MT trial, in which patients were randomized to thrombectomy with concurrent intravenous alteplase vs. EVT alone. Thrombectomy is known to be more efficient in recanalizing vessel occlusions, and as such, reperfusion rates were much higher compared with previous studies that did not include thrombectomy patients and rather focused on medical treatment regimens [10]. In our study, the adverse effect of hyperglycemia may have become more predominant, since the detrimental effect of hyperglycemia is known to be more pronounced in the setting of brain reperfusion [2, 4, 17].
Implication for Clinical Practice and Future Studies
The results of our study may have direct implications for intravenous alteplase decision-making in large vessel occlusion patients who are eligible for intravenous alteplase treatment and directly present to an endovascular thrombectomy-capable hospital. The lack of benefit of intravenous alteplase treatment in hyperglycemic patients suggests that it may be beneficial to forego alteplase administration in hyperglycemic patients, while in patients without evidence of hyperglycemia, intravenous alteplase should routinely be considered.
In addition, the results of our study raise the question whether strict glucose management in patients receiving endovascular treatment would improve clinical outcome. However, a series of studies have investigated this question in detail and showed no benefit of attempts to lower blood glucose using insulin [31, 32], and one should also be cautious to avoid hypoglycemia when trying to correct elevated blood glucose levels. Some other studies found certain anti-diabetic drugs, such as glibenclamide and other sulfonylurea agents, have promising neuroprotection effect in stroke patients besides lowering the blood glucose [33, 34]. As such, administration such drugs seems an alternative method for patients with hyperglycemia. However, controversies still existed. The efficacy and safety of intravenous glibenclamide are being evaluated by a phase 3 study in patients with severe cerebral edema following large hemispheric infarction, which may provide more evidence of the clinical usage of such drugs [35].
Strengths and Limitations
The major strength of our study is the randomized comparison of thrombectomy alone versus bridging therapy in a group of patients eligible for concurrent intravenous alteplase administration and endovascular thrombectomy.
However, our study has some limitations. First, as a post-hoc analysis, there is a risk of type I errors due to multiple testing [36]. Furthermore, when continuous variables were dichotomized, statistical power may be reduced. This may potentially contribute to the non-significant treatment-by-hyperglycemia interaction in the unadjusted analysis regarding to the primary outcome, while treatment-by-glucose level (continuous variable) interaction existed in the analysis. However, the cut-point (7.8 mmol/L) was based on clinical justification, and dichotomization provided an applicable approach for clinical practice. Second, the blood glucose measurement method can influence blood glucose levels. For example, plasma glucose values are approximately 11% higher than whole blood glucose levels when the hematocrit is normal, and postprandial capillary exceed venous blood glucose levels by up to 20% [37]. We used blood plasma glucose in our study, and hence, our results may not be generalizable to blood glucose measurements when using another approach. In addition, the percentage of patients with hypoglycemia (≤ 3.9 mmol/L) and extremely high blood glucose (> 22.2 mmol/L) was low, which could affect our estimation in these patients. Moreover, as a random blood glucose, the reasons causing hyperglycemia vary. For example, some patients may have been stomached while others not. Studies are needed to explore whether the heterogeneity would affect the results in this study. Third, we did not record the longitudinal course of blood glucose during the hospital stay and at follow-up visits. Neither the use of lowering glucose drugs was documented. Whether and how the longitudinal course of blood glucose and the use of anti-hyperglycemic drugs would influence the detrimental effect of hyperglycemia warrants further investigation [38]. Fourth, our study sample consisted exclusively of large vessel occlusion patients presenting directly to a thrombectomy-capable hospital. As such, they may not be generalizable to transfer patients and to those with medium or small vessel occlusions.
Conclusions
For large vessel occlusion patients undergoing endovascular thrombectomy at the presenting hospital, hyperglycemia modified alteplase treatment effect on clinical outcome. Combination therapy with thrombectomy and concurrent intravenous alteplase may be preferred in patients without hyperglycemia, while in hyperglycemic patients, it may be reasonable to withhold intravenous alteplase and proceed with thrombectomy alone. However, it is of note this post-hoc study is exploratory. Studies from other trials that randomized combination therapy vs thrombectomy alone, and possibly subsequent trials targeting the hyperglycemic patients after stroke, are needed to confirm our study.
References
Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59(5):669–74.
Alvarez-Sabin J, Molina CA, Ribo M, et al. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke. 2004;35(11):2493–8.
Broocks G, Kemmling A, Aberle J, et al. Elevated blood glucose is associated with aggravated brain edema in acute stroke. J Neurol. 2020;267(2):440–8.
Mandava P, Martini SR, Munoz M, et al. Hyperglycemia worsens outcome after rt-PA primarily in the large-vessel occlusive stroke subtype. Transl Stroke Res. 2014;5(4):519–25.
Suh SW, Shin BS, Ma H, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64(6):654–63.
Ribo M, Molina C, Montaner J, et al. Acute hyperglycemia state is associated with lower tPA-induced recanalization rates in stroke patients. Stroke. 2005;36(8):1705–9.
Saqqur M, Shuaib A, Alexandrov AV, et al. The correlation between admission blood glucose and intravenous rt-PA-induced arterial recanalization in acute ischemic stroke: a multi-centre TCD study. Int J Stroke. 2015;10(7):1087–92.
Chamorro A, Brown S, Amaro S, et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke. 2019;50(3):690–6.
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–418.
Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without Intravenous Alteplase in Acute Stroke. N Engl J Med. 2020;382(21):1981–93.
Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP Randomized Clinical Trial. JAMA. 2021;325(3):244–53.
Zi W, Qiu Z, Li F, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT Randomized Clinical Trial. JAMA. 2021;325(3):234–43.
LeCouffe NE, Kappelhof M, Treurniet KM, et al. A randomized trial of intravenous alteplase before endovascular treatment for stroke. N Engl J Med. 2021;385(20):1833–44.
Fischer U, Kaesmacher J, S Plattner P, et al. SWIFT DIRECT: Solitaire with the intention for thrombectomy plus intravenous t-PA versus DIRECT Solitaire Stent-retriever thrombectomy in acute anterior circulation stroke: methodology of a randomized, controlled, multicentre study. Int J Stroke. 2022;17(6):698–705.
Ribo M, Montaner J, Molina CA, et al. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke. 2004;35(9):2123–7.
Alvarez-Sabin J, Molina CA, Montaner J, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke. 2003;34(5):1235–41.
Yang P, Treurniet KM, Zhang L, et al. Direct Intra-arterial thrombectomy in order to Revascularize AIS patients with large vessel occlusion Efficiently in Chinese Tertiary hospitals: A Multicenter randomized clinical Trial (DIRECT-MT)-Protocol. Int J Stroke. 2020;15(6):689–98.
Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31.
Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
Goyal M, Fargen KM, Turk AS, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6(2):83–6.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7.
von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke. 2015;46(10):2981–6.
Kruyt ND, Biessels GJ, Devries JH, Roos YB. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6(3):145–55.
Nordt TK, Klassen KJ, Schneider DJ, Sobel BE. Augmentation of synthesis of plasminogen activator inhibitor type-1 in arterial endothelial cells by glucose and its implications for local fibrinolysis. Arterioscler Thromb. 1993;13(12):1822–8.
Mistry EA, Mistry AM, Nakawah MO, et al. Mechanical Thrombectomy Outcomes With and Without Intravenous Thrombolysis in Stroke Patients: A Meta-Analysis. Stroke. 2017;48(9):2450–6.
Bruno A, Biller J, Adams HP, Jr., et al. Acute blood glucose level and outcome from ischemic stroke. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Neurol. 1999;52(2):280–4.
Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002;105(14):1679–85.
Poppe AY, Majumdar SR, Jeerakathil T, et al. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32(4):617–22.
Piironen K, Putaala J, Rosso C, Samson Y. Glucose and acute stroke: evidence for an interlude. Stroke. 2012;43(3):898–902.
Johnston KC, Bruno A, Pauls Q, et al. Intensive vs Standard Treatment of Hyperglycemia and Functional Outcome in Patients With Acute Ischemic Stroke: The SHINE Randomized Clinical Trial. JAMA. 2019;322(4):326–35.
Gray CS, Hildreth AJ, Sandercock PA, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007;6(5):397–406.
Sheth KN, Elm JJ, Molyneaux BJ, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15(11):1160–9.
Sheth KN, Petersen NH, Cheung K, et al. Long-Term Outcomes in Patients Aged </=70 Years With Intravenous Glyburide From the Phase II GAMES-RP Study of Large Hemispheric Infarction: An Exploratory Analysis. Stroke. 2018;49(6):1457–63.
Biogen. Phase 3 study to evaluate the efficacy and safety of intravenous BIIB093 (glibenclamide) for severe cerebral edema following large hemispheric infarction (CHARM). Available at: https://www.clinicaltrialsgov/ct2/show/NCT02864953. Acessed 7 Jul 2022.
Srinivas TR, Ho B, Kang J, Kaplan B. Post hoc analyses: after the facts. Transplantation. 2015;99(1):17–20.
Kim HS. Blood glucose measurement: is serum equal to plasma? Diabetes Metab J. 2016;40(5):365–6.
Kim TJ, Lee JS, Park SH, Ko SB. Short-term glycemic variability and hemorrhagic transformation after successful endovascular thrombectomy. Transl Stroke Res. 2021.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
The trial was funded by the Stroke Prevention Project of the National Health Commission of the People’s Republic of China and the Wu Jieping Medical Foundation. The analysis of this post-hoc analysis was partially funded by SanHang Program of the Naval Military Medical University and “Climbing” program of Changhai Hospital. The design and data collection were performed by members of the executive committee and the local investigators of each participating center. The steering committee had the final responsibility for the decision to submit the paper for publication. The study sponsors were not involved in the study design, study conduct, protocol review, or manuscript preparation or review.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosures
Dr Goyal reports compensation from MicroVention, Inc. for consultant services; compensation from Mentice for consultant services; compensation from Stryker for consultant services; grants from Medtronic to other; compensation from Medtronic for consultant services; and grants from Johnson & Johnson Health Care Systems Inc. Dr Ospel also reports compensation from NICOLab (unrelated to the manuscript) for consultant service.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu Zhou, Zijun Wang, Johanna Ospel are co-first authors. Professors Hongxin Han and Tong Li are co-correspoding authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Wang, Z., Ospel, J. et al. Effect of Admission Hyperglycemia on Safety and Efficacy of Intravenous Alteplase Before Thrombectomy in Ischemic Stroke: Post-hoc Analysis of the DIRECT-MT trial. Neurotherapeutics 19, 1932–1941 (2022). https://doi.org/10.1007/s13311-022-01281-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-022-01281-0