Abstract
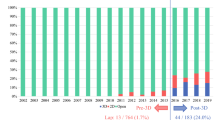
Three-dimensional visualization technology (3DVT) has been recently introduced to achieve a precise preoperative planning of liver surgery. The aim of this observational study was to assess the accuracy of 3DVT for complex liver resections. 3DVT with hyper accuracy three-dimensional (HA3D™) technology was introduced at our institution on February 2020. Anatomical characteristics were collected from two-dimensional imaging (2DI) and 3DVT, while intraoperative and postoperative outcomes were recorded prospectively. A total of 62 patients were enrolled into the study. 3DVT was able to study tumor extension and liver anatomy, identifying at least one vascular variation in 37 patients (59.7%). Future remnant liver volume (FRLV) was measured using 2DI and 3DVT. The paired samples t test assessed positive correlation between the two methods (p < 0.001). At least one vessel was suspected to be invaded by the tumor in 8 (15.7%) 2DI cases vs 16 (31.4%) 3DVT cases, respectively. During surgery, vascular invasion was detected in 17 patients (33.3%). A total of 73 surgical procedures were proposed basing on 2DI, including 2 alternatives for 16 patients. After 3DVT, the previously planned procedure was changed in 15 cases (29.4%), due to the clearer information provided. A total of 51 patients (82%) underwent surgery. The most frequent procedure was right hepatectomy (33.3%), followed by left hepatectomy (23.5%) and left trisectionectomy (13.7%). Vascular resection and reconstruction were performed in 10 patients (19.6%) and portal vein was resected in more than half of these cases (66.7%). 3DVT leads to a more detailed and tailored approach to complex liver surgery, improving surgeons’ knowledge of liver anatomy and accuracy of liver resection.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three-dimensional (3D) technology has been recently introduced in clinical surgical practice. Specifically, in liver cancer surgery the precision of preoperative imaging is extremely important for diagnosis, for precise tumor localization, for estimation of resectability and to provide an accurate resection planning. The application of the most innovative technologies to liver surgery may allow to improve results, with increased accuracy and outcomes of liver resections. Among these, 3D visualization technology (3DVT) is an important field of research and it is gradually becoming an essential tool for hepatobiliary surgeons. Particularly, 3DVT is able to display, describe and explain morphology and spatial distribution of liver segments, biliary tree and blood vessels [1]. New additive manufacturing technologies with precise 3D print (3DP) of the liver and 3D intraoperative navigation (3DIN) systems are recent and innovative tools applied for visualization of 3D model. The application of the virtual environment during surgery using 3DIN allows the visualization in real time, improving the spatial orientation of surgeons and recall anatomical details during surgical procedure [2].
The utilization of 3DVT is useful in liver surgery for the complex segmental anatomy, the high frequency of individual variations and the complex relationship between tumors and main anatomical structures [2].
The application of 3DVT was proposed also to improve doctor-patient communication and to help patients in understanding the complexity of the disease and of planned surgical procedure [1].
Furthermore, 3DVT may facilitate the learning process of both medical students and residents, the precise spatial mapping of liver segments, the ability to locate the tumor. Tumor relationships with surrounding vascular and biliary structures are more easily identified by 3D technologies [3,4,5]. Consequently, a better comprehension of liver anatomy may improve surgical residents’ skills in both minimally invasive and open liver surgery [6].
In addition, the best knowledge of patient’s liver anatomy and tumor localization may improve the intraoperative confidence of the surgeon, leading to reduction of surgical time, number of complications, length of hospital stay and costs [7].
However, the utilization of 3DVT in liver surgery is recent, it needs validation from the scientific society and is still under evaluation in literature [8].
This study aimed to describe the results of a preliminary experience with 3DVT and 3DIN application to complex liver resections in a single center of hepatobiliary surgery.
Methods
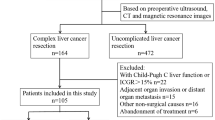
This prospective observational monocentric study was conducted at the Unit of Hepato-biliary surgery of the University of Verona from February 2020 to December 2021. The patients who were eligible for complex liver surgery were included, and 3DVT and 3DIN were applied for complex open and minimally invasive liver resections.
Preoperative characteristics
The main demographic and clinical characteristics of patients were recorded during the preoperative medical examination, such as: gender, age, body mass index (BMI), comorbidities, liver function assessed by the evaluation of indocyanine green retention rate at 15 min (ICG R15), preoperative blood exams and preoperative diagnosis. In all cases future remnant liver volume (FRLV) of the planned resection was assessed using the computed tomography (CT) scans (two-dimensional imaging, 2DI) before the application of 3DVT. FRLV was expressed as a ratio of FRLV and total functioning liver volume (TLV). Liver volumetry was measured using a processing software from the 2DI. Liver volume calculation for 2DI was performed with standard procedure outlining liver, tumor and resection margins to calculate total liver volume, tumor volume and segmental planned remnant liver volumetry.
Portal vein embolization (PVE) was performed when the FRLV was lower than 40% of TLV, following the criteria previously published [9]. In these cases, the CT scans acquired 3–4 weeks after PVE were used for 3DVT. Liver volumetry after PVE was recorded to calculate the hypertrophy ratio using the following formula: hypertrophy ratio = 100 × (FRLV after PVE – FRLV before PVE)/FRLV before PVE [10].
The information collected from the 2DI reports was recorded, in particular: number of lesions, tumor size, tumor location, relationship between tumor and major vessels if available.
3D reconstruction of liver anatomy
Hyper accuracy 3D reconstructions with Hyper accuracy three-dimensional (HA3D™) technology of the images in DICOM format were processed by a dedicated software (MEDICS, Moncalieri, Turin, Italy). This technology was introduced at our institution in February 2020.
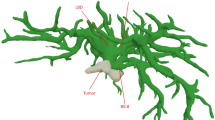
During the preoperative planning, complex cases of liver tumors were reconstructed to collect useful information on tumor location, anatomical variations of the main liver vessels and tumor involvement of biliary and vascular structures (Fig. 1). The definition of complex liver resection was based on the following characteristics: central tumor localization, suspicious vascular involvement, need for extended hepatectomy with vascular resection and reconstruction, biliary involvement by the tumor, and suspicious locally advanced disease.
3D visualization technology of a case of peri-hilar cholangiocarcinoma. a Tumor extension (orange) to the right hepatic duct and left hepatic duct (segment 4 origin, narrow); b contact between the lesion (orange) and right hepatic artery (narrow); c contact between the lesion (orange) and right portal vein (narrow); d future remnant liver volumetry of right trisectionectomy
The 3DVT was used during the preoperative planning to decide the most appropriate treatment in each case and intraoperatively to guide the surgeon during liver resection (Fig. 2).
A case of left trisectionectomy preserving the dorsal biliary duct for segment 8 and the corresponding parenchyma for Bismuth-Corlette type IV peri-hilar cholangiocarcinoma. a 3D simulation of left trisectionectomy preserving the dorsal biliary duct for segment 8 (B8d) in addition to bile ducts for segment 6 (B6) and 7 (B7) and the corresponding parenchyma; b 3D printed model of the resection; c, d intraoperative image of remnant liver (segment 8 dorsal, S8d; segment 7, S7; segment 6, S6) and hilar structures after resection
The virtual simulation software allowed the surgeon to navigate each patient’s anatomy and hide the different structures of the liver as appropriate, to identify the exact location of the tumor and its proximity to blood vessels or biliary branches. Furthermore, virtual simulation of the resection and 3D liver volumetry helped the surgeon during the preoperative planning.
The 3D liver volumes were measured studying either portal perfusion and hepatic veins’ drainage, and included: TLV, tumor volume (TV), planned resection volume and FRLV (Fig. 1D).
Blood vessels’ anatomical characteristics and variations
Vessels’ anatomical characteristics and variations were categorized according to the classifications previously published in literature [11,12,13,14,15,16].
The Michels’ classification of hepatic artery anatomy described 10 types of variations: type I, normal pattern; type II, replaced left hepatic artery (LHA) from left gastric artery (LGA); type III, replaced right hepatic artery (RHA) from superior mesenteric artery (SMA); type IV, replaced LHA and RHA (types II + III); type V, accessory LHA from LGA; type VI, accessory RHA from SMA; type VII, accessory LHA and RHA (types V + VI); type VIII, accessory LHA and replaced RHA (types V + III); IX type, replaced common hepatic artery from SMA; type X, replaced common hepatic artery from LGA [13].
Portal vein anatomy according to the Couinaud’s classification may vary into 5 types: type I, normal pattern; type II or portal vein trifurcation, the main portal vein (MPV) is divided into right anterior (RAPV), right posterior (RPPV) and left portal veins (LPV); type III, the RPPV arises directly from MPV as its first branch, at the lower part of hepatic hilum, and LPV is the terminal branch, arising after the origin of the RAPV; type IV or trifurcation of right portal vein (RPV), segment 7 branch is the first branch of RPV; type V, segment 6 branch arises early as first branch of RPV [14, 16].
The hepatic veins’ drainage was mapped in detail by Tani et al., who classified the major tributaries as following: tributaries of left hepatic vein, such as left superficial vein and umbilical fissure vein; tributaries of middle hepatic vein, such as superior or inferior vein for segment 4 (V4sup and V4inf, respectively), ventral or intermediate vein for segment 8 (V8v and V8i, respectively), and veins for segment 5; tributaries of right hepatic vein, such as right superficial vein and dorsal vein for segment 8; accessory veins, such as inferior right hepatic vein (IRHV) and middle right hepatic vein [15, 17]. Most of those tributaries are usually too thin to be clearly observed at the CT scans, hence in this study only the veins with a diameter greater than 3 mm were considered major tributaries and recorded [17].
Finally, the relationship between the tumor and the major vessels was assessed by the 2DI, 3DVT and intraoperatively, basing on the characteristics of vessels’ shape and diameter [1, 12, 18].
Intraoperative and postoperative outcomes
The surgical procedures were performed by four expert hepatobiliary surgeons (AG, AR, TC, SC).
The intraoperative data were collected from surgical reports, including type of liver resection, tumor-vessels’ relationship and vascular resections. The nomenclature of hepatectomies was defined according to the Brisbane 2000 terminology of liver resections [19].
The intraoperative navigation device ICON (Intraoperative COgnitive Navigation system, MEDICS, Moncalieri, Turin, Italy), was applied for intraoperative 3DVT evaluation (Fig. 3). The ICON device allowed the surgeon to manipulate 3DVT using a touchless system during surgery, to establish the tumor extension, identify the principal vessels, help achieving the oncological radicality and decreasing the risk of complications. The postoperative outcomes were recorded prospectively, including postoperative complications, length of hospital stay (LOS) and death within 30 days from surgery. The comprehensive complication index was calculated to assess the global morbidity of each patient [20].
Statistical analysis
The software SPSS (Version 25.0, Chicago, IL) was used to carry out the statistical analysis. The continuous variables were expressed as median and the corresponding interquartile range (IQR) or mean ± standard deviation (SD). The categorical variables were expressed as counts and percentages. The paired samples t-test was used to analyze the correlation between 2DI and 3DVT measurements of FRLV. The Bland–Altman plot was applied to assess the agreement between the 2DI and the 3DVT, as two different methods of FRLV measurement. Then, the regression linear analysis was performed to reveal the grade of agreement. The p value ≤ 0.05 established the statistical significancy.
Results
A total of 62 cases were reconstructed by 3DVT and enrolled into the study. The clinical data of each patient were collected in a prospective database from February 2020 to December 2021. During this period, 51 patients (82%) underwent surgery and the postoperative short-term outcomes were recorded.
Preoperative characteristics
The main preoperative characteristics are reported in Table 1. Among the 62 cases included into the study, the 61.3% (n = 38) were men and the median age of the patients was 69.0 years old (IQR, 60.5–76.5). The median BMI was 25.0 kg/m2 (IQR, 22.2–27.8). More than half of the patients had comorbidities increasing the operative risks, according to the American Society of Anesthesiologists’ physical status classification system (ASA score ≥ 3, 54.7%). The most frequent diagnosis was peri-hilar cholangiocarcinoma (PHCC) (n = 30, 48.4%), followed by intrahepatic cholangiocarcinoma (iCCC) (n = 12, 19.3%), hepatocellular carcinoma (HCC) (n = 10, 16.1%), gallbladder cancer (GBC), colorectal liver metastases (CRLM) (both n = 4, 6.4%) and primary sclerosing cholangitis (PSC) (n = 2, 3.2%). The median number of lesions detected was 1 (range, 1–7) and the median tumor size was 30.0 mm (IQR, 19.5–72.5). PBD was necessary in the 40.3% of the patients (n = 25) to relieve jaundice. The most frequent method of PBD was PTBD (n = 19). The ICG R15 assessment of liver function showed a mean value of 9.8% (± 7.7). PVE was performed in the 16.1% of patients (n = 10), being the FRLV lower than 40% of TLV. The hypertrophy ratio after PVE was 51.0% (IQR, 35.8–129.0) and the median time from PVE to surgery was 36 days (IQR, 33.5–49.7). The median time from 2DI and 3DVT was 24 days (IQR, 11–41). While the median time from 3DVT and surgery was 8 days (IQR, 2–25).
3D visualization technology description of vascular anatomy
Descriptive data of the 3DVT are reported in Table 2. The 3DVT identified at least one vascular variation in 37 patients (59.7%) and 21 of them (56.7%) had more than one variation. In particular, the 3DVT assessed 63 variations of the hepatic artery anatomy. According to Michels classification the most frequent variations were type III and V (both 19.0%, n = 12), followed by type VI (4.8%, n = 3), type IX (3.2%, n = 2). A total of 33 (52.4%) variations were not described by the classification and the most frequent of them was the presence of LHA arising from celiac trunk (n = 7, 11.1%) and accessory LHA from proper hepatic artery (n = 5, 7.9%).
A total of 9 patients (14.5%) had variation of portal vein anatomy, in particular: type I, 2 patients (22.2%); type II, 3 patients (33.3%); type III, 1 patient (11.1%) and type IV, 1 patient (11.1%), 2 patients (22.2%) had non-classified variations.
Eventually, major hepatic venous tributaries (≥ 3 mm in diameter) were detected by 3DVT in a total of 32 patients (51.6%) and 4 (12.5%) patients of them had more than one major tributary. In particular, a major inferior right hepatic vein was assessed in 30 cases (83.3%), only 1 patient had both major inferior vein for segment 4 and major ventral vein for segment 8. Furthermore, the presence of intrahepatic venous shunts was evident in 5 cases (13.9%).
The 2DI identified at least one vascular variation in 31 cases (50.0% of patients), while 3DVT described vascular variations in 37 cases (59.7% of patients). The same variations were found by the two methods. The 2DI was able to identify vascular variations in most cases with an agreement between the two modalities of 83.7%.
Surgical planning
The data of surgical planning are reported in Table 3. According to liver volumetry assessed using the CT scans, the median FRLV of the resection planned was 51.0% (IQR, 38.7–73.8), while the FRLV of the alternative resection was 39.4% (29.2–56.4). The median FRLV of the first resection proposed was 51.0% (IQR, 40.0–75.0) and it was 43.0% (IQR, 31.5–58.5) for the alternative planned resection.
At the paired samples t-test comparing the measurements of the FRLV assessed using 2DI and 3DVT, the two methods were significantly positively correlated (r = 0.781, p < 0.001).
Furthermore, the Bland–Altman plot was performed to compare the use of 2DI and 3DVT for estimation of FRLV (Fig. 4) and they did not achieve the statistically significance (p = 0.897), confirming the good agreement between the two methods of FRLV estimation.
After a multidisciplinary evaluation of each case, a total of 71 surgical procedures were proposed, with 2 different alternatives in 13 cases. However, after the 3DVT evaluation, the comprehension of tumor location and extension was more precise and the previously procedure planned was changed in 15 cases (29.4%). In particular, in 4 cases (7.8%) the disease was locally advanced and patients were candidates for neoadjuvant therapy, while in 4 cases (7.8%) the resection was modified from extended hepatectomy to major, in 2 cases (3.9%) it was modified from major hepatectomy to extended, in 1 case (2.0%) from major to minor resection and in 1 case (2.0%) from the anatomical resection into a non-anatomical one.
The preoperative evaluation of tumor-vessels’ relationship revealed a higher number of suspected vascular invasions at the 3DVT in comparison with 2DI (31.4% vs 15.7%, respectively). This result was similar to the intraoperative evidence, where the surgeon found at least one vessel invaded in 17 patients (33.3%). Hence, while 2DI had a greater specificity than 3DVT [91.2% (95% CI, 76.3–98.1) vs 80.6% (95% CI, 64.0–91.8), respectively], 3DVT had a greater sensitivity than 2DI [52.9% (95% CI, 27.8–77.0) vs 29.4% (95% CI, 10.3–56.0), respectively].
Intraoperative and postoperative outcomes
The intraoperative data and the postoperative outcomes are reported in Table 4. A total of 51 (82.3%) patients underwent liver resection after 3DVT application. The most frequent surgical procedure was right hepatectomy (33.3%, n = 17), followed by left hepatectomy (23.5%, n = 12), minor resections (21.6%, n = 11), left trisectionectomy (13.7%, n = 7), right trisectionectomy and mesohepatectomy (both 3.9%, n = 2). Vascular resection and reconstruction were performed in 10 patients (19.6%), PV was the most frequent resected vessel (66.7%, n = 8).
The median tumor size was 36.5 mm (IQR, 20.0–72.5) and the paired samples t test confirmed good correlation of tumor size measurement among histological examination and both 3DVT (r = 0.936, p < 0.001) and 2DI (r = 0.862, p < 0.001).
The median global postoperative morbidity assessed by comprehensive complication index (CCI) was 29.6 (IQR, 20.9–40.8). The median length of hospital stay (LOS) was 10 days (IQR, 7–17). A total of 3 patients died within 30 days from surgery (5.9%).
Discussion
In the field of liver cancer surgery, the accurate preoperative radiological findings allow to predict technical resectability. The application of the modern 3D technology may add further details to 2DI and improve surgical outcomes. In particular, the precise description of tumor location, tumor extension, pattern of tumor borders and its relationships with surrounding structures may greatly influence resectability and type of surgical procedure [21,22,23].
In literature, a good correspondence between 3DVT and surgical specimen was clearly demonstrated. Yang et al. compared 3DVT, 3DP and 2DI of patients undergoing liver resection for oncological disease. The Authors found a significant improvement in the accuracy of tumor location and preoperative planning of resection using 3D technology, in particular 3DP [6]. Lopez et al. analyzed the accuracy and usefulness of 3D printed models (3DP) applied to liver surgery, learning curve and doctor–patient communication. In particular, the Authors reported a good agreement between 3D technologies and 2DI in terms of measurement of vessels’ and bile ducts’ calibers and their distances from tumor. Also, resection margins predicted by the 3DP were accurate and comparable with the surgical specimen [3]. Among methods displaying 3DVT, we applied an innovative device for intraoperative navigation that allowed the surgeon to manipulate 3DVT during surgery allowing a more precise resection.
Liver anatomy is characterized by high variability among the individuals. A precise definition of liver segments’ portal and arterial supply, and venous drainage may allow to plan more accurate anatomic liver resections. In addition to the information provided by the 2DI, the 3DVT ability to reconstruct and intuitively display vessels may improve significantly the preoperative planning based on the morphological features of blood vessels [17, 24].
The 3DVT provided a great number of detailed information on the vessels’ anomalies, their course and relationship with the tumor. We identified 63 variations of hepatic artery, in 37 patients (59.7%), 21 of them (56.7%) with multiple variations. Evidence of major venous variants was described by the 3DVT in 32 patients (51.6%).
The 3DVT demonstrated to be more accurate than 2DI in the identification of liver lesions, which are displayed in the different spatial projections, hence it may reduce some subjective errors during preoperative planning and evaluation of vascular invasion [24]. Zhang et al. published their experience with the 3DVT applied to diagnosis of portal vein invasion during the preoperative planning of PHCC. The Authors reported an increased sensitivity, specificity and overall accuracy of 3DVT visualization in comparison with the standard 2DI evaluation [25]. In the present study the evaluation of tumor involvement of blood vessels was performed by expert radiologists and surgeons, who found a better agreement between 3DVT and surgery than 2DI. We observed tumor invasion of at least one vessel in 8 (15.7%) patients’ 2DI, in 16 (31.4%) patients’ 3DVT and 17 (33.3%) patients’ intraoperative findings. The precision of representing tumor–vessels’ relationships using 3DVT may reduce unnecessary vascular dissections or resections, decreasing considerably the risk of complications, such as bleeding or devascularization of biliary tree [8].
The accuracy of 3DVT in displaying liver and vessels anatomy, tumor relationship with the surrounding structures and measurement of FRLV allows the surgeons to plan a more accurate liver resection and be more confident during surgery, due to the previous detailed comprehension of patients’ anatomy [7]. Witowski et al. underlined the relevant role of 3DVT during the preoperative planning influencing the resection proposal [26]. In our experience the type of resection planned after the evaluation of 2DI was changed by the surgeon after the 3DVT in 29.4% of the cases (n = 15) because of the clearer information provided. In literature, the indications for application of 3DVT to liver surgery have not been well established. It increases the costs, and the majority of the studies focus on the technical aspects instead of the quantitative statistical and clinical results [5, 27]. Nowadays, the most reasonable indication seems to apply these technologies to complex cases with high risk of complications [23].
The results of this preliminary study have some limitations that should be addressed.
Firstly, the sample size is small because of the recent introduction of the 3D technology at our institution. Secondly, in this preliminary study we included different types of liver tumor. A study regarding the 3DVT application to a single complex disease is planned to achieve more precise and focused results and identify the 3DVT added value on the postoperative short- and long-term outcomes.
Thirdly, the evaluation of tumor-vessels’ involvement was subjective, but performed by expert radiologists and surgeons. However, in future the application and validation of a specific 3DVT artificial intelligence algorithm could increase the precision of 3D technology.
Conclusions
In conclusion, the application of 3D technology to liver surgery is promising, because it may improve surgeons’ perception of liver vascular and biliary anatomy, tumor extension and relationship with main liver structures. All of these aspects may enhance the accuracy of preoperative resection planning, prediction of intraoperative complications and of postoperative outcomes. Hence, the application of 3DVT to complex liver resections is certainly promising, although it needs larger population and validation studies focused on the clinical outcomes.
References
Fang C, An J, Bruno A, Cai X, Fan J, Fujimoto J et al (2020) Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int 14(4):437–453. https://doi.org/10.1007/s12072-020-10052-y
Huber T, Huettl F, Tripke V, Baumgart J, Lang H (2021) Experiences with three-dimensional printing in complex liver surgery. Ann Surg 273(1):e26–e27. https://doi.org/10.1097/sla.0000000000004348
Lopez-Lopez V, Robles-Campos R, García-Calderon D, Lang H, Cugat E, Jiménez-Galanes S et al (2021) Applicability of 3D-printed models in hepatobiliary surgey: results from “LIV3DPRINT” multicenter study. HPB (Oxford) 23(5):675–684. https://doi.org/10.1016/j.hpb.2020.09.020
Kong X, Nie L, Zhang H, Wang Z, Ye Q, Tang L et al (2016) Do three-dimensional visualization and three-dimensional printing improve hepatic segment anatomy teaching? A randomized controlled study. J Surg Educ 73(2):264–269. https://doi.org/10.1016/j.jsurg.2015.10.002
Perica ER, Sun Z (2018) A systematic review of three-dimensional printing in liver disease. J Digit Imaging 31(5):692–701. https://doi.org/10.1007/s10278-018-0067-x
Yang T, Lin S, Xie Q, Ouyang W, Tan T, Li J et al (2019) Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg Endosc 33(2):411–417. https://doi.org/10.1007/s00464-018-6308-8
Pereira da Silva N, Abreu I, Serôdio M, Ferreira L, Alexandrino H, Donato P (2020) Advanced hepatic vasculobiliary imaging segmentation and 3D reconstruction as an aid in the surgical management of high biliary stenosis. BMC Med Imaging. 20(1):120. https://doi.org/10.1186/s12880-020-00520-0
Wang Y, Cao D, Chen SL, Li YM, Zheng YW, Ohkohchi N (2021) Current trends in three-dimensional visualization and real-time navigation as well as robot-assisted technologies in hepatobiliary surgery. World J Gastrointest Surg 13(9):904–922. https://doi.org/10.4240/wjgs.v13.i9.904
Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C (2012) How much remnant is enough in liver resection? Dig Surg 29(1):6–17. https://doi.org/10.1159/000335713
Kasai Y, Hatano E, Iguchi K, Seo S, Taura K, Yasuchika K et al (2013) Prediction of the remnant liver hypertrophy ratio after preoperative portal vein embolization. Eur Surg Res 51(3–4):129–137. https://doi.org/10.1159/000356297
Fang C, Zhang Y, Fan Y, Yang J, Xiang N, Zeng N (2014) Three-dimensional reconstruction of individual hepatic veins and portal veins system in hepatectomy. Zhonghua Wai Ke Za Zhi 52(1):45–49
Chinese S, Liver C, Clinical P, Digital I (2020) [Clinical practice guidelines for precision diagnosis and treatment of complex liver tumor guided by three-dimensional visualization technology (version 2019)]. Nan Fang Yi Ke Da Xue Xue Bao 40(3):297–307. https://doi.org/10.12122/j.issn.1673-4254.2020.03.01
Noussios G, Dimitriou I, Chatzis I, Katsourakis A (2017) The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature. J Clin Med Res 9(4):248–52. https://doi.org/10.14740/jocmr2902w
Chen W, Zhao L, Wang J, Guo WL (2020) Hepatic vascular variations and visual three-dimensional reconstruction technique in pediatric patients with choledochal cyst. Surg Radiol Anat 42(12):1489–1499. https://doi.org/10.1007/s00276-020-02590-9
Tani K, Shindoh J, Akamatsu N, Arita J, Kaneko J, Sakamoto Y et al (2016) Venous drainage map of the liver for complex hepatobiliary surgery and liver transplantation. HPB (Oxford) 18(12):1031–1038. https://doi.org/10.1016/j.hpb.2016.08.007
Couinaud C (1999) Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 16(6):459–467. https://doi.org/10.1159/000018770
Zhang J, Guo X, Qiao Q, Zhao J, Wang X (2021) Anatomical study of the hepatic veins in segment 4 of the liver using three-dimensional visualization. Front Surg 8:702280. https://doi.org/10.3389/fsurg.2021.702280
Zhu W, He SS, Zeng SL, Zhang P, Yang J, Xiang N et al (2019) Three-dimensional visual assessment and virtual reality study of centrally located hepatocellular carcinoma on the axis of blood vessels. Zhonghua Wai Ke Za Zhi 57(5):358–365. https://doi.org/10.3760/cma.j.issn.0529-5815.2019.05.008
Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY et al (2000) The Brisbane 2000 terminology of liver anatomy and resections. HPB 2(3):333–339. https://doi.org/10.1016/S1365-182X(17)30755-4
Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA (2013) The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 258(1):1–7. https://doi.org/10.1097/SLA.0b013e318296c732
Bartsch F, Hahn F, Müller L, Baumgart J, Hoppe-Lotichius M, Kloeckner R et al (2021) Intrahepatic cholangiocarcinoma: Introducing the preoperative prediction score based on preoperative imaging. Hepatobiliary Pancreat Dis Int 20(3):262–270. https://doi.org/10.1016/j.hbpd.2020.08.002
Zhu LY, Hou JC, Yang L, Liu ZR, Tong W, Bai Y et al (2022) Application value of mixed reality in hepatectomy for hepatocellular carcinoma. World J Gastrointest Surg 14(1):36–45. https://doi.org/10.4240/wjgs.v14.i1.36
Sheng W, Yuan C, Wu L, Yan J, Ge J, Lei J (2021) Clinical application of a three-dimensional reconstruction technique for complex liver cancer resection. Surg Endosc. https://doi.org/10.1007/s00464-021-08636-2
Banchini F, Romboli A, Rizzi N, Luzietti E, Conti L, Capelli P (2021) Laparoscopic dorsal subsegmentectomy 8: Exploit the 3d technology to plan liver resection, and predict intraparenchymal pedicles. A case report. (With video explanation). Int J Surg Case Rep 88:106516. https://doi.org/10.1016/j.ijscr.2021.106516
Zhang J, Guo X, Wang H, Zhang J, Liu P, Qiao Q et al (2020) The application of three-dimensional visualization in preoperative evaluation of portal vein invasion in hilar cholangiocarcinoma. Cancer Manag Res 12:9297–9302. https://doi.org/10.2147/cmar.S264479
Witowski J, Budzyński A, Grochowska A, Ballard DH, Major P, Rubinkiewicz M et al (2020) Decision-making based on 3D printed models in laparoscopic liver resections with intraoperative ultrasound: a prospective observational study. Eur Radiol 30(3):1306–1312. https://doi.org/10.1007/s00330-019-06511-2
Witowski JS, Coles-Black J, Zuzak TZ, Pędziwiatr M, Chuen J, Major P et al (2017) 3D Printing in liver surgery: a systematic review. Telemed J E Health 23(12):943–947. https://doi.org/10.1089/tmj.2017.0049
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. This study was funded by University of Verona Joint project 2019 Grant. The preliminary results of this study were included in an e-poster presented at the 15th IHPBA World Congress in New York.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests to disclose.
Compliance with Ethical Standards
This study was conducted in accordance with Ethical Standards.
Research involving human participants and/or animals
This article does not contain any research involving human participants or animals.
Informed consent
All patients signed informed consent at the preoperative visit.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruzzenente, A., Alaimo, L., Conci, S. et al. Hyper accuracy three-dimensional (HA3D™) technology for planning complex liver resections: a preliminary single center experience. Updates Surg 75, 105–114 (2023). https://doi.org/10.1007/s13304-022-01365-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-022-01365-8