Abstract
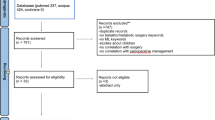
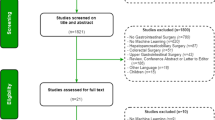
Artificial intelligence (AI), including machine learning (ML), is being slowly incorporated in medical practice, to provide a more precise and personalized approach. Pancreatic surgery is an evolving field, which offers the only curative option for patients with pancreatic cancer. Increasing amounts of data are available in medicine: AI and ML can help incorporate large amounts of information in clinical practice. We conducted a systematic review, based on PRISMA criteria, of studies that explored the use of AI or ML algorithms in pancreatic surgery. To our knowledge, this is the first systematic review on this topic. Twenty-five eligible studies were included in this review; 12 studies with implications in the preoperative diagnosis, while 13 studies had implications in patient evolution. Preoperative diagnosis, such as predicting the malignancy of IPMNs, differential diagnosis between pancreatic cystic lesions, classification of different pancreatic tumours, and establishment of the correct management for each of these lesions, can be facilitated through different AI or ML algorithms. Postoperative evolution can also be predicted, and some studies reported prediction models for complications, including postoperative pancreatic fistula, while other studies have analysed the implications for prognosis evaluation (from predicting a textbook outcome, the risk of metastasis or relapse, or the mortality rate and survival). One study discussed the possibility of predicting an intraoperative complication—massive intraoperative bleeding. Artificial intelligence and machine learning models have promising applications in pancreatic surgery, in the preoperative period (high-accuracy diagnosis) and postoperative setting (prognosis evaluation and complication prediction), and the intraoperative applications have been less explored.

Similar content being viewed by others
Availability of data and materials
Study data are available at request from the corresponding author.
References
Hashimoto DA, Ward TM, Meireles OR (2020) The role of artificial intelligence in surgery. Adv Surg 54:89–101. https://doi.org/10.1016/j.yasu.2020.05.010
Xiang Y, Zhao L, Liu Z et al (2020) Implementation of artificial intelligence in medicine: status analysis and development suggestions. Artif Intell Med 102:101780. https://doi.org/10.1016/j.artmed.2019.101780
Ramesh A, Kambhampati C, Monson J, Drew P (2004) Artificial intelligence in medicine. Ann R Coll Surg Engl 86:334–338. https://doi.org/10.1308/147870804290
McGuigan A, Kelly P, Turkington RC et al (2018) Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 24:4846–4861. https://doi.org/10.3748/wjg.v24.i43.4846
Bednar F, Simeone DM (2014) Recent advances in pancreatic surgery. Curr Opin Gastroenterol 30:518–523. https://doi.org/10.1097/MOG.0000000000000096
Torphy RJ, Fujiwara Y, Schulick RD (2020) Pancreatic cancer treatment: better, but a long way to go. Surg Today 50:1117–1125. https://doi.org/10.1007/s00595-020-02028-0
Hashimoto DA, Rosman G, Rus D, Meireles OR (2018) Artificial intelligence in surgery: promises and perils. Ann Surg 268:70–76. https://doi.org/10.1097/SLA.0000000000002693
Zhou X-Y, Guo Y, Shen M, Yang G-Z (2020) Application of artificial intelligence in surgery. Front Med 14:417–430. https://doi.org/10.1007/s11684-020-0770-0
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. https://doi.org/10.1371/journal.pmed.1000097
Page MJ, McKenzie JE, Bossuyt PM et al (2021) Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol 134:103–112. https://doi.org/10.1016/j.jclinepi.2021.02.003
Cui S, Tang T, Su Q et al (2021) Radiomic nomogram based on MRI to predict grade of branching type intraductal papillary mucinous neoplasms of the pancreas: a multicenter study. Cancer Imaging 21:26. https://doi.org/10.1186/s40644-021-00395-6
Kuwahara T, Hara K, Mizuno N, et al (2019) Usefulness of Deep Learning Analysis for the Diagnosis of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Clinical and Translational Gastroenterology 10: 1–8. https://doi.org/10.14309/ctg.0000000000000045
Kang JS, Lee C, Song W et al (2020) Risk prediction for malignant intraductal papillary mucinous neoplasm of the pancreas: logistic regression versus machine learning. Sci Rep 10:20140. https://doi.org/10.1038/s41598-020-76974-7
Si K, Xue Y, Yu X et al (2021) Fully end-to-end deep-learning-based diagnosis of pancreatic tumors. Theranostics 11:1982–1990. https://doi.org/10.7150/thno.52508
Tonozuka R, Itoi T, Nagata N et al (2021) Deep learning analysis for the detection of pancreatic cancer on endosonographic images: a pilot study. J Hepatobiliary Pancreat Sci 28:95–104. https://doi.org/10.1002/jhbp.825
Park S, Chu LC, Hruban RH et al (2020) Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging 101:555–564. https://doi.org/10.1016/j.diii.2020.03.002
Springer S, Masica DL, Dal Molin M et al (2019) A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med 11:4772. https://doi.org/10.1126/scitranslmed.aav4772
Yang J, Guo X, Ou X et al (2019) Discrimination of pancreatic serous cystadenomas from mucinous cystadenomas with CT textural features: based on machine learning. Front Oncol 9:494. https://doi.org/10.3389/fonc.2019.00494
Watson MD, Lyman WB, Passeri MJ et al (2021) Use of artificial intelligence deep learning to determine the malignant potential of pancreatic cystic neoplasms with preoperative computed tomography imaging. Am Surg 87:602–607. https://doi.org/10.1177/0003134820953779
Wei R, Lin K, Yan W et al (2019) Computer-aided diagnosis of pancreas serous cystic neoplasms: a radiomics method on preoperative MDCT images. Technol Cancer Res Treat 18:153303381882433. https://doi.org/10.1177/1533033818824339
Machicado JD, Chao W-L, Carlyn DE et al (2021) High performance in risk stratification of intraductal papillary mucinous neoplasms by confocal laser endomicroscopy image analysis with convolutional neural networks (with video). Gastrointest Endosc 94:78-87.e2. https://doi.org/10.1016/j.gie.2020.12.054
Zhou RQ, Ji HC, Liu Q et al (2019) Leveraging machine learning techniques for predicting pancreatic neuroendocrine tumor grades using biochemical and tumor markers. WJCC 7:1611–1622. https://doi.org/10.12998/wjcc.v7.i13.1611
Han IW, Cho K, Ryu Y et al (2020) Risk prediction platform for pancreatic fistula after pancreatoduodenectomy using artificial intelligence. World J Gastroenterol 26:4453–4464. https://doi.org/10.3748/wjg.v26.i30.4453
Klimov S, Xue Y, Gertych A et al (2021) Predicting metastasis risk in pancreatic neuroendocrine tumors using deep learning image analysis. Front Oncol 10:593211. https://doi.org/10.3389/fonc.2020.593211
Kambakamba P, Mannil M, Herrera PE et al (2020) The potential of machine learning to predict postoperative pancreatic fistula based on preoperative, non-contrast-enhanced CT: a proof-of-principle study. Surgery 167:448–454. https://doi.org/10.1016/j.surg.2019.09.019
Merath K, Hyer JM, Mehta R et al (2020) Use of machine learning for prediction of patient risk of postoperative complications after liver, pancreatic, and colorectal surgery. J Gastrointest Surg 24:1843–1851. https://doi.org/10.1007/s11605-019-04338-2
Cos H, Li D, Williams G et al (2021) Predicting outcomes in patients undergoing pancreatectomy using wearable technology and machine learning: prospective Cohort Study. J Med Internet Res 23:e23595. https://doi.org/10.2196/23595
Sahara K, Paredes AZ, Tsilimigras DI et al (2021) Machine learning predicts unpredicted deaths with high accuracy following hepatopancreatic surgery. Hepatobiliary Surg Nutr 10:20–30
Skawran SM, Kambakamba P, Baessler B et al (2021) Can magnetic resonance imaging radiomics of the pancreas predict postoperative pancreatic fistula? Eur J Radiol 140:109733. https://doi.org/10.1016/j.ejrad.2021.109733
Palumbo D, Mori M, Prato F et al (2021) Prediction of early distant recurrence in upfront resectable pancreatic adenocarcinoma: a multidisciplinary, machine learning-based approach. Cancers 13:4938. https://doi.org/10.3390/cancers13194938
Li X, Yang L, Yuan Z et al (2021) Multi-institutional development and external validation of machine learning-based models to predict relapse risk of pancreatic ductal adenocarcinoma after radical resection. J Transl Med 19:281. https://doi.org/10.1186/s12967-021-02955-7
Zhang Y, Zhu S, Yuan Z et al (2020) Risk factors and socio-economic burden in pancreatic ductal adenocarcinoma operation: a machine learning based analysis. BMC Cancer 20:1161. https://doi.org/10.1186/s12885-020-07626-2
Kaissis GA, Jungmann F, Ziegelmayer S et al (2020) Multiparametric modelling of survival in pancreatic ductal adenocarcinoma using clinical, histomorphological, genetic and image-derived parameters. J Clin Med 9:1250. https://doi.org/10.3390/jcm9051250
Lee K-S, Jang J-Y, Yu Y-D et al (2021) Usefulness of artificial intelligence for predicting recurrence following surgery for pancreatic cancer: Retrospective cohort study. Int J Surg 93:106050. https://doi.org/10.1016/j.ijsu.2021.106050
Wakiya T, Ishido K, Kimura N et al (2021) Prediction of massive bleeding in pancreatic surgery based on preoperative patient characteristics using a decision tree. PLoS ONE 16:e0259682. https://doi.org/10.1371/journal.pone.0259682
Bradley A, van der Meer R, McKay C (2019) Personalized pancreatic cancer management. Pancreas 48:598–604. https://doi.org/10.1097/MPA.0000000000001312
Bari H, Wadhwani S, Dasari BVM (2021) Role of artificial intelligence in hepatobiliary and pancreatic surgery. WJGS 13:7–18. https://doi.org/10.4240/wjgs.v13.i1.7
Takaori K (2017) “Revisions of the International consensus Fukuoka guidelines for the management of IPMN of the pancreas”: progress for twelve years. Pancreatology 17:645–646. https://doi.org/10.1016/j.pan.2017.08.008
del Chiaro M, Verbeke C, Salvia R et al (2013) European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 45:703–711. https://doi.org/10.1016/j.dld.2013.01.010
Gonzalo-Marin J (2014) Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. WJGO 6:360–368. https://doi.org/10.4251/wjgo.v6.i9.360
Wani S, Hall M, Keswani RN et al (2015) Variation in aptitude of trainees in endoscopic ultrasonography, based on cumulative sum analysis. Clin Gastroenterol Hepatol 13:1318-1325.e2. https://doi.org/10.1016/j.cgh.2014.11.008
Zaheer A, Singh V, Akshintala V et al (2014) Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography. J Comput Assist Tomogr 38:146–152. https://doi.org/10.1097/RCT.0b013e3182a9a431
Schorn S, Demir IE, Vogel T et al (2019) Mortality and postoperative complications after different types of surgical reconstruction following pancreaticoduodenectomy—a systematic review with meta-analysis. Langenbeck’s Arch Surg 404:141–157. https://doi.org/10.1007/s00423-019-01762-5
Lyu Y, Li T, Cheng Y et al (2018) Pancreaticojejunostomy versus pancreaticogastrostomy after pancreaticoduodenectomy: an up-to-date meta-analysis of RCTs applying the ISGPS (2016) criteria. Surg Laparosc Endosc Percutaneous Techniq 28:139–146. https://doi.org/10.1097/SLE.0000000000000530
Hüttner FJ, Fitzmaurice C, Schwarzer G et al (2016) Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Datab Syst Rev 2016:CD006053. https://doi.org/10.1002/14651858.CD006053.pub6
Chua IS, Gaziel-Yablowitz M, Korach ZT et al (2021) Artificial intelligence in oncology: path to implementation. Cancer Med 10:4138–4149. https://doi.org/10.1002/cam4.3935
Funding
“Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca—Financing Contract number 1032/60/13 January 2021 for Doctoral research projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Standards
Not applicable.
Research involving human participants and/or animals
Not applicable, the study is a systematic review.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schlanger, D., Graur, F., Popa, C. et al. The role of artificial intelligence in pancreatic surgery: a systematic review. Updates Surg 74, 417–429 (2022). https://doi.org/10.1007/s13304-022-01255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-022-01255-z