Abstract
Multi-drug resistant organisms (MDR-Os) are emerging as a significant cause of surgical site infections (SSI), but clinical outcomes and risk factors associated to MDR-Os-SSI have been poorly investigated in general surgery. Aims were to investigate risk factors, clinical outcomes and costs of care of multi-drug resistant organisms (MDR-Os-SSI) in general surgery. From January 2018 to December 2019, all the consecutive, unselected patients affected by MDR-O SSI were prospectively evaluated. In the same period, patients with non-MDR-O SSI and without SSI, matched for clinical and surgical data were used as control groups. Risk factors for infection, clinical outcome, and costs of care were compared by univariate and multivariate analysis. Among 3494 patients operated on during the study period, 47 presented an MDR-O SSI. Two control groups of 47 patients with non-MDR-O SSI and without SSI were identified. MDR-Os SSI were caused by poly-microbial etiology, meanly related to Gram negative Enterobacteriales. MDR-Os-SSI were related to major postoperative complications. At univariate analysis, iterative surgery, open abdomen, intensive care, hospital stay, and use of aggressive and expensive therapies were associated to MDR-Os-SSI. At multivariate analysis, only iterative surgery and the need of total parenteral and immune-nutrition were significantly associated to MDR-Os-SSI. The extra-cost of MDR-Os-SSI treatment was 150% in comparison to uncomplicated patients. MDR-Os SSI seems to be associated with major postoperative complications and reoperative surgery, they are demanding in terms of clinical workload and costs of care, they are rare but increasing, and difficult to prevent with current strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Healthcare associated infections (HAI) cause significant morbidity and mortality in subjects admitted to acute care hospitals [1,2,3,4]. The Center for Disease Control and Prevention (CDC) reported in the USA, between 2011 and 2014, 365,490 HAI caused by central line-associated bloodstream infections (CLABSI) [23.5%], catheter associated urinary tract infections (CAUTI) [37.8%], ventilator-associated pneumonia (VAP) [2.2%], and surgical site infections (SSI) [36.4%]. About 50% of the SSI were related to abdominal surgery, with prevalence depending on the type of operation [1]. The European Center for Disease Control and Prevention (ECDC) reported the prevalence of HAI in Europe as high as 3.4%, with 4.2 million HAIs in 2013 [2]. Even though HAI decreased in the last decade, a shift from naïve bacteria to drug-resistant organisms has been observed, resulting in a worse prognosis for patients and increased costs for the Health Systems [1, 3, 5, 6]. Staphylococcus aureus and Escherichia coli are the most frequently isolated bacteria from SSI culture, with a resistance rate ranging from 42.7 to 44.7% for S. aureus, and from 13.3 to 15.3% for E. coli [1].
Prior hospitalization, previous antibiotic treatments and preoperative infections are risk factors causing antibiotic resistance [7,8,9]. As proposed by the ECDC, bacteria should be classified as multi-drug resistant (MDR-Os), extended-drug resistant (XDR-Os), and pan-drug resistant organisms (PDR-Os) when they have developed resistance to one or two, three or more, or to all known antibiotics respectively [10]. MDR-Os infection has been studied in patients undergoing transplantation, oncologic, and emergency surgery [11,12,13,14,15,16,17,18,19,20,21], but if resistance increases morbidity and mortality in the general population [6, 22], it is not clear if it plays the same role in surgical populations [12, 17,18,19].
The aim of the present study was to investigate, in surgical patients affected by SSI, the risk factors and the effects of MDR-Os infections, and to quantify the extra-costs for their treatment.
Patients and methods
From January 2017 to December 2019, following the indication of the National health authorities’ program against antibiotics resistance (PNCAR) [23], a systematic microbiologic investigation of all patients affected by SSI was prospectively performed, to assess incidence, risk factors, impact on clinical course, and healthcare costs of MDR-Os related infections in surgical patients. SSI was defined as any infection occurred within 30 days after the operation and classified as superficial, deep or organ-space according to Culver [24]. Follow-up included an outpatient visit within 30 days after the hospital discharge. The study was submitted to and approved by the Ethic Committee of the L. Sacco Hospital (#00-28004), and an informed consent was obtained from all the patients. The study was conducted according to the ethical standards of the Declaration of Helsinki (2013 version) and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] guidelines [25, 26].
Study design
The aim of the study was to evaluate the effects of MDR-Os infection on the clinical course of patients undergoing general surgery. Primary endpoints were morbidity and mortality, classified according to Clavien–Dindo. Grade 1 and 2 were considered minor complications, grade 3–4 major complications, and grade 5 corresponds to the death of the patient [27]. Secondary endpoints were the identification of risk factors for MDR-Os infection and determination of the extra-costs caused by MDR-Os infections. Major complications were very frequent in patients affected by MDR-Os SSI (> 50%) and very rare in patients with non-MDR-Os SSI (< 10%) or without SSI (≈ 1%). From the nomogram of Feigl, with a 95% confidence limit and interval of 50% a number of observations = 35 in each group was considered sufficient for the analysis. Therefore, we decided to maintain the number of 47 observations and to include two control groups: one group of patients affected by non-MDROs SSI and a second of patients without SSI. Control groups were selected in the same time frame of MDR-Os patients, and standardized by age, male/female ratio, diagnosis, and surgical treatment.
The following preoperative risk factors were considered for the analysis: prior hospitalization within 12 months; prior antibiotic or immunosuppressive therapy ( Steroid, azathioprine, mercaptopurine or infliximab and analogs) within 3 months; preoperative hospital stay (days); body mass index (BMI [weight kg/height m2]) < 18.5 or > 30; weight loss > 5% in the last 3 months or > 10% without time limits; Type 2 diabetes mellitus (basal plasma glucose > 120 mg/dL and glycated hemoglobin > 6.5%); American Society of Anesthesiology (ASA) Physical Status > 2; blood values of total protein (g/L), albumin (g/L), transthyretin (TST) (mg/dL), C-reactive protein (mg/L), red blood cell count (RBC 1012/L), hemoglobin (g/L), white blood cell count (WBC 109/L), and lymphocyte count (LC 109/L). Peri-operative surgical data considered in the study were: indication for surgery; elective or emergency setting; open or laparoscopic approach; pre-existing infections; surgical wound classification (clean, clean-contaminated, contaminated, dirty-infected, according to Mangram [28]); duration of the surgery (min); abdominal drainage insertion; open abdomen treatment; and iterative surgery (number of reoperations). To evaluate the clinical course of the patients affected by MDR-Os, the following postoperative parameters were registered: duration of postoperative hospital stay (days); total hospital stay (days); surgical (SSI, dehiscence, fistula, hemorrhage, occlusion) and non-surgical (cardio-vascular and respiratory) complication rate; non-SSI complications rate (CLABSI, CAUTI and VAP cases were recorded if positive microbiological cultures were obtained); the type, posology and duration of the antibiotic treatment; admission to the Intensive Care Unit (ICU) and its duration (days); parenteral and/or enteral nutrition; blood transfusion; naso-gastric tube; central venous catheter; urinary catheter for more than 3 days. Evolution from infection to sepsis, septic shock and organ failure was recorded according to the Third International Consensus definitions for sepsis and septic shock (Sepsis-3), using the quick sequential organ failure assessment score (qSOFA) > 2 as threshold criteria [29].
Microbiology
All the patients received broad-spectrum antibiotic prophylaxis except patients with infection at time of surgery who had empiric interval antibiotic therapy until cultures and antibiotic Minimum Inhibiting Concentration (MIC) were available. Patients with SSI or septic collection underwent culture of fluid sample, blood, urine, bronchoalveolar lavage and invasive devices when indicated. Semi-quantitative and qualitative cultures were plated in standard microbiological media and incubated for 48 h both aerobically and anaerobically. Antimicrobial susceptibility was determined by the disk diffusion technique. In selected cases, the MIC gradient strip test (Etest, bioMérieux, France) was performed following the European Committee on Antimicrobial Testing (EUCAST) recommendations [28]. The main categories of resistance were: methicillin resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium (VRE), extended spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella species, Pseudomonas aeruginosa and Acinetobacter species resistant to third generation cephalosporins and carbapenems, Carbapenem resistant Enterobacteriales (CRE).
Analysis of the MDR-Os SSI induced costs
To quantify the extra-cost of MDR-Os infections we considered the reimbursement obtained by the National Health System, based on the diagnosis-related group classification system (DRG), which calculates the diagnosis, the surgical procedure and the occurrence of complications [30]. The reimbursement is increased by a diary cost until a threshold length of stay is reached; thereafter the charge per diem is reduced. The cost of the antibiotic therapy was determined on the basis of the price established by the Italian national drug authority (AIFA) for each dose of the drug [31].
Statistical analysis
Differences among groups were calculated using a two-tailed, Fischer’ exact test for categorical variables, and a Student t or Mann–Whitney U test where appropriate. Binary and multinomial logistic regression were also performed. To conduct the multivariate statistical analyses, biochemical continuous variables were transformed into categorical variables using laboratory reference cut-off values. The duration of TPN, INT, ICU stay, and hospitalization were not considered in the multivariate analysis. Preoperative stay was considered in case of more than one night spent in the hospital before the surgical intervention. Results are reported as OR with 95% confidence interval. Values of p < 0.05 were considered significant. Statistical analysis was performed using IBM SPSS Statistics v.25.
Results
From January 2018 to December 2019, a total of 3494 operations were performed at the Second Unit of General Surgery of Luigi Sacco University Hospital. Abdominal procedures were 1970, and clean extra-abdominal procedures 1524. There were 472 cases of Inflammatory Bowel Disease (IBD), 355 gastro-intestinal cancer cases, 158 bariatric patients, 700 miscellaneous operations (mainly cholecystectomy and laparoscopic hernias), and 285 emergency surgeries. SSI rate was 4.43% (155 cases) and 47 patients presented an MDR-Os SSI, with an overall incidence of 1.34%. The highest rate was registered after emergency surgery (14/285 cases, 4.91%), when infection was present at the time of operation; IBD surgery had the second rank with 17/472 cases (3.64%), followed by oncologic surgery (10/355 cases, 2.81%), and bariatric surgery (3/158 cases, 1.8%). Miscellaneous operations had the lowest incidence of MDR-Os infection (3/700 cases, [0.42%]). There was a significant difference between clean and other surgeries (p < 0.00001), but not among the other abdominal procedures. MDR-Os patients had a mean age of 59.8 ± 17.4, a M/F ratio of 1.23, and the same surgical indications of the control groups, but there were more elective surgeries (72.3%), laparotomic approach (61.7%) and contaminated-dirty operations (59.5%). In Table 1 are reported the differences among groups in terms of surgical parameters. MDR-Os SS had more reoperation rate and open abdomen treatment, while patients without SSI had a significantly shorter operation time, more laparoscopic procedures and clean or clean-contaminated operations, less use of abdominal drains, and no iterative surgery or open abdomen.
Several clinical and biochemical findings resulted as risk factors associated to SSI (Table 2). In the multinomial logistic regression analysis, the only variable that reached statistical significance between MDR-O and non-MDR-O patients was transthyretin. MDR-O SSI patients were characterized by a significant presence of comorbidities, and transthyretin levels < 0.20 mg/dL in comparison to patients without SSI. The length of hospital stay in patients without SSI, with non-MDR, and with MDR-SSI was 10.19 ± 5.2, 18.3 ± 8.2, and 47.8 ± 42 days respectively.
In Table 3 are reported the univariate and the multivariate analysis of peri-operative complications. In the multivariate analysis, MDR-O SSI postoperative course was characterized by a higher reoperation rate and complications (Clavien–Dindo 3 and 4). An association between multi-drug resistance and multi-microbial infection was the most predictive factor of high-grade surgical complications. Furthermore, patients with MDR-O SSI had a higher rate of dehiscence and organ-space SSI. This increased the risk of further complications after surgery, such as reoperations, septic shock, and multi organ failure.
During the postoperative course, summarized in Table 4, MDR-Os infected patients needed blood transfusions and Mechanical Assisted Ventilation (MAV) more frequently than non-MDRO-SSI patients, and were the only to receive a tracheostomy. TPN use and duration was significantly higher in MDR-Os SSI patients in comparison to non-MDR-Os SSI and non-infected patients. Immuno-nutritional therapy (INT) was adopted more frequently in MDR-O SSI patients than non-MDR-O patients, but without a significant difference in duration. Patients affected by MDR-Os infections were admitted to ICU more frequently, and for a longer time than non-MDR-O SSI patients. The total postoperative course duration and the hospital stay were significantly longer in the MDR-Os SSI patients. By the multivariate regression analysis only a significant implementation of artificial nutrition, in particular INT and TPN, was evident in MDR-O-SSI patients.
The mean cost of treatment for patients without SSI was 8965 ± 6611 euros (total cost 421,382 euros), 12,202 ± 3533 euros (total cost 573,499 euros) in the presence of SSI (p < 0.05), and 22,473 ± 19,749 (total cost 1,056,275 euros) when the patient developed an MDR-Os SSI (p < 0.01). The cost of the antibiotic therapy, based on the AIFA prices in Italy, was 300 euros for the group of patients without SSI, 9700 euros for the group with SSI, and 115,000 euros for the group affected by MDR-O SSI.
Microbiology
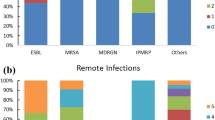
Patients affected by non-MDR-Os SSI had more Gram negative Enterobacteriales (61%) than Gram positive (30.5%) cultures. Escherichia coli (36.3%) was the most frequently isolated bacterium, followed by Enterococcus faecium (9.09%), Morganella morganii (7.58%), Staphylococcus aureus (6%) and Pseudomonas aeruginosa (6%). Yeastes (Candida albicans) were isolated in two cases (3%) (Fig. 1a). In patients with MDR-Os SSI, Staphylococcus species (MRSA) were isolated in 21 (27.6%) cases, Escherichia coli (ESBL, CRE) in 20 (26.1%), Klebsiella pneumoniae (ESBL, CRE)in 17 (22%), and Pseudomonas aeruginosa (MDR) in 6 (7.89). Enterococcus species (VRE), Acinetobacter baumani (MDR) and Candida glabrata (MDR) were isolated in a limited number of cases (Fig. 1b). Extended spectrum β-Lactamase resistance was the most frequent type (31.5%), followed by methicillin resistance (27.6%), carbapenem resistance (18.4%) and vancomycin resistance Enterococcus sp. (11.8%). Patients affected by MDR-Os were 43 (91%), by XDR-Os were 3 (6.3%), and only 1 was PDR-Os.
Microbiology of non-MDR-Os (A) and MDR-Os (B) cultures. A The first graph represents the class distribution of non-MDR pathogens in our sample. Gram+ Cocci: S. aureus (6.06%), S. epidermidis (1.51%), S. parasanguinis (1.51%), S. salivaris (1.51%), E. faecalis (7.57%), E. faecium (9.09%), E. casseliflavus (1.51%). Gram + Bacilli: B. cereus (1.51%). Enterobacterales: E. coli (36.36%), Klebsiella spp. (9,08%), E. cloacae (3.03%), P. mirabilis (1.51%), S. marcescens (3.03%), M. morganii (7.57%). Non-fermenting Gram−: A. baumannii (6.06%). Fungi: C. albicans (3.03%). B The second one represents the distribution of MDR pathogens based on the type of antimicrobial resistance. Gram+ Cocci (blue shades): MRSA (10.52%); MR Cocci with S. hominis (3.94%), S. haemolyticus (9.21%), S. epidermidis (2.63%), S. capitis (1.31%), S. mitis (1.31%); VRE with E. faecalis (2.63%), E. faecium (7.89%). Enterobacterale (orange shades): ESBL + with E. coli (21.05%), K. pneumoniae (7.89%); CRE with E. coli (3.94%), K. pneumoniae (14.47%); VRE with E. coli (1.31%). Non-fermenting Gram−: A. baumannii (2.63%), P. aeruginosa (7.89%). Fungi: C. glabrata (1.31%). MRSA methycillin-resistant Staphylococcus aureus; VRE vancomycin-resistant Enterococci, ESBL+ extended spectrum beta-lactamase
Multi-bacterial infections were more frequent in MDR-Os versus non-MDR-OS patients (38.3% versus 14.8%, p < 0.01), were found at the first culture of biologic sample in four cases (22.2%), and emerged in 14 cases during antibiotic therapy, as effect of the antibiotic pressure on the bacterial resistoma. The association between multi-drug resistance and multi-bacterial infection was the most predictive factor of major surgical complications: 12/18 (66%) patients with this characteristic had grade 4 and 5 Clavien–Dindo complications.
Discussion
This is a prospective, case–control study, focused on the treatment of MDR SSI in general surgery.
The main drawback of the study is that it is a single center study and it comes from a large University Hospital of a highly industrialized urban area. However, MDROs-SSI are probably under-reported by general surgeons, since nearly 1/3 of the SSI infections in our series were related to MDR-Os, a very high incidence that should be considered with alarm.
Resistance to the antibiotics is a typical characteristic of bacteria. It might be pre-existent and activated by antibiotics, transferred from resistant to nonresistant bacteria as long as the infection and its causes persist, and enhanced by the suppression of sensible with relative expansion resistant strains. Thus, the pathways of MDR-Os infection are multifactorial and several clinical conditions are able to trigger these effects. Previous administration of immunosuppressive agents [32], antibiotics, and hospital admission [8, 9, 19] are reported risk factors for MDR-Os expansion. However, in our series other known factors for SSI were associated to MDR-O-SSI: emergency operation [33], presence of abscess and dirty operations [34], oncologic surgery [15, 16], IBD surgery [35, 36], and visceral anastomosis [13]. An elevated ASA score, obesity, and malnutrition (low levels or plasma proteins, albumin and transthyretin) were also associated to MDR-Os infection [37, 38]. Furthermore, the degree of MDR-Os infection was all the higher, the longer was the antibiotic treatment used to overcome the original infection: in our series only 8.5% of the MDR-Os-SSI infections was poly-microbial at presentation, but a further 29.7% became MDR-Os poly-microbial during the clinical course. The development of new MDR-Os was related to the length of the antibiotic treatment and the number of the antibiotics used. However, extended or pan-drug resistance were rare and did not cause a worsening of the prognosis, as reported in the field of hepato-biliary, lung and hepatic transplantation surgery [17,18,19].
Only culture-guided use of antibiotics can reduce inappropriate prescription and the risk of MDR-Os development [39,40,41,42], but a further approach could be to recognize patients at risk of MDR-Os before culture, to reduce risk factors. Unfortunately, risk factors for MDR and non-MDR infections are similar, and their correction often requires a longer hospital stay which in turn is another risk factor for MDR infections (e.g. as it is in our series for low transthyretin levels associated to MDR-Os-SSI in multivariate analysis).
Looking to surgical factors associated to MDRO-S-SSI, it is always difficult to distinguish between causes and effects of the infection. We know that the risk of SSI is higher after dirty operations [34], in emergency [33] and after open laparotomy [24, 43]. In selecting patients for the control group, we have offset these factors and found that MDR-Os-SSI was associated to major surgical complications (according to Clavien–Dindo class III–V), suture dehiscence and organ-space SSI, with a significantly higher use of resources and reoperation rate. The microbiology of infected patients demonstrated a high frequency of Gram-positive Cocci (39 vs 29%) and a low frequency of Gram negative Enterobacteriales (48.6 vs 61%) in MDR-Os SSI in comparison to non-MDR-Os SSI. Extended spectrum β-Lactamase resistance was found more frequently (31.5%) than methicillin resistance in Staphylococcus sp. (27.6%), Carbapenem resistance of Enterobacteriales (18.4%) and vancomycin resistance of Enterococcus sp. In the first decade of twenty-first century, MRSA Staphylococcus aureus was the most diffuse multi-drug resistant bacteria [42], but in the last 10 years, according to our experience, there were several observations [15, 16, 18,19,20,21, 32] of MDR-Os SSI caused by Gram negative bacteria, bearing ESBL, VRE and CRE resistance. According to the classification of Majiorakos [10], the majority of antibiotic-resistant bacteria in our series were MDR (90%), and only 10% were XDR or PDR (one patient), but this microbiologic profile could be sufficient to reduce the efficacy of standard short-term antibiotic prophylaxis [14], and of empiric interval therapy [21] started before the results of microbiologic cultures. Albeit the best antibiotic therapy, the infection persists until the barrier between the external world and the “milieu interieur” is restored. To achieve this effect, we need to support the immune response, to correct malnutrition, and to avoid dehiscence by the use of ostomies in a septic condition. Takahashi et al. [13] demonstrated that MDR-Os SSI were more frequent when hepatectomy was combined with biliary tract resection (39.1% against 15.8%), due to the risk associated to bilio-intestinal anastomosis.
Finally, the treatment of MDR-Os patients had elevated costs in terms of duration of hospitalization, antibiotic therapy, and critical care [6]. Hospitalization costs increased by 36% in non-MDR-Os SSI and by 150.6% in MDR-Os SSI patients. The increase in costs for antibiotic therapy was 0.07% in patients without SSI, 1.59% in non-MDR-Os SSI, and 10.88% in MDR-Os SSI. Costs, in this series, were calculated on the basis of the Italian National Health System reimbursement program, but they could also be considered in other countries with different programs.
In conclusion, MDR-Os SSI are increasing, seem to be associated with deep protein malnutrition, major postoperative complications, suture dehiscence and iterative surgery; they are difficult to prevent, demanding in terms of clinical workload and costs of care and need a wise surgical program.
Change history
17 July 2022
Missing Open Access funding information has been added in the Funding Note.
Abbreviations
- SSI:
-
Surgical site infections
- MDR-Os:
-
Multi-drug resistant organisms
- XDR-Os:
-
Extended-drug resistant organisms
- PDR-OS:
-
Pan-drug resistant organisms
- HAI:
-
Healthcare associated infections
- CDC:
-
Center for Disease Control
- ECDC:
-
European Center for Disease Control
- CLABSI:
-
Central line-associated bloodstream infections
- CAUTI:
-
Catheter associated urinary tract infections
- VAP:
-
Ventilator-associated pneumonia
- TPN:
-
Total parenteral nutrition
- INT:
-
Immuno-nutritional therapy
- ICU:
-
Intensive care unit
- qSOFA:
-
Quick sequential organ failure assessment score
- MIC:
-
Minimum inhibiting concentration
- MRSA:
-
Methicillin resistant Staphylococcus aureus
- VRE:
-
Vancomycin resistant Enterococcus faecium
- ESBL:
-
Extended spectrum beta-lactamase
- CRE:
-
Carbapenem resistant Enterobacteriales
- IBD:
-
Inflammatory bowel diseases
- MAV:
-
Mechanical assisted ventilation
References
Weiner LM, Webb AK, Limbago B et al (2016) Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the Centers for Diseases Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37:1288–1301
ECDC (2014) Annual epidemiological report. Antibiotic resistance and healthcare-associated infections. http://www.ecdc.europa.eu
McDonald LC (2016) Vital signs: preventing antibiotic-resistant infections in Hospitals United States, 2014. Am J Transplant 16:2224–2230
Cassini A, Diaz-Hogberg L, Plachouras D et al (2019) Attributable deaths and disability-adjusted life years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 19:56–66
CDC (2015) The five “W”s of the targeted assessment for prevention (TAP) strategy, Atlanta, GA: US Department of Health and Human Services. CDC. http://www.cdc.gov/hai/prevent/tap.html
Cosgrove SE, Carmeli Y (2003) The impact of antimicrobial resistance on health and economic outcomes. CID 36:1433–1437
Wright GD (2007) The antibiotic resistoma nexus of chemical and genetic diversity. Nat Rev Microbiol 5:175–186
Nseir S, Grailles G, Soury-Lavergne A et al (2010) Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug-resistant bacteria at intensive-care unit admission. Clin Microbiol Infect 16:902–908
Sun L, Liu S, Wang J, Wang L (2019) Analysis of risk factors for multiantibiotic-resistant infections among surgical patients at a Children’s hospital. Microb Drug Resist 25:297–303
Magiorakos AP, Srinivasan A, Carag RB et al (2012) Multidrug-resistant, extensively drug-resistant and pan drug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Viehman JA, Clancy CJ, Clarke L et al (2016) Surgical site infections after liver transplantation: emergence of multidrug-resistant bacteria and implications for prophylaxis and treatment strategies. Transplantation 100:2107–2114
Sugawara G, Yokoyama Y, Ebata T et al (2018) Preoperative biliary colonization/infection caused by multidrug-resistant (MDR) pathogens in patients undergoing major hepatectomy with extrahepatic bile duct resection. Surgery 163:1106–1113
Takahashi Y, Takesue Y, Frejiwara M et al (2018) Risk factors for surgical site infection after major hepatobiliary surgery and pancreatic surgery. J Infect Chemother 24:739–743
Sano S, Sugiura T, Kawamura I et al (2019) Third-generation cephalosporin for antimicrobial prophylaxis in pancreatoduodenectomy in patients with internal preoperative biliary drainage. Surgery 165:559–564
Golzarri MF, Sanchez JS, Cornejo-Juarez P et al (2019) Colonization by fecal extended-spectrum β-lactamase-producing Enterobacteriaceae and surgical site infections in patients with cancer undergoing gastrointestinal and gynecological surgery. Am J Infect Control 47:916–921
Hernaiz-Leonardo JC, Golzarri MF, Cornejo-Juarez P et al (2017) Microbiology of surgical site infections in patients with cancer: a 7-year review. Am J Infect Cont 45:761–766
Dobbin C, Maley M, Harkness J et al (2004) The impact of pan-resistant bacterial pathogens on survival after lung transplantation in cystic fibrosis: results from a single large referral center. J Hosp Infect 56:277–282
Augustin P, Kermarrec N, Muller-Serieys C et al (2010) Risk factors for multidrug resistant bacteria and optimization of empirical antibiotic therapy in postoperative peritonitis. Crit Care 14 (article no. R20)
Seguin P, Fedun Y, Laviolle B et al (2010) Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J Antimicrob Chemother 65:342–346
Di Carlo P, Gulotta G, Caruccio A et al (2013) KPC-3 Klebsiella pneumonia ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: analysis of a case series of 30 patients. BMC Anesthesiol 13(1):13
Montravers P, Dufour G, Guglielminetti J et al (2015) Dynamic changes of microbioal flora and therapeutic consequences in persistent peritonitis. Crit Care 19:70 (1–13)
Friedman ND, Temkin E, Carmeli Y (2016) The negative impact of antibiotic resistance. Clin Microbiol Infect 22:416–422
PNCAR. National Action Plan on Antimicrobial Resistance 2017–20 (2017). http://www.salte.gov.it/images/PNCAR. Accessed 1 Nov 2021
Culver DH, Horan TC, Gaynes RP et al (1991) Surgical wound infection rates by wound class, operative procedure, and patient risk index National Nosocomial Infections Surveillance System. Am J Med 91:152S-157S
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457
World Medical A (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191–2194
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
EUCAST Recommendations (2017). http://www.eucast.org/clinical-breakpoints/. Accessed 1 Nov 2021
Singer M, Deutschman CS, Seymour CW et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315:801–810
DRG TIP TAR_SD OMDCD (2015). https://www.regione.lombardia.it. Accessed 1 Nov 2021
AIFA. Agenzia Italiana del Farmaco. Prezzi e rimborso farmaci di classe A e H (2020). http://aifa.gov.it/liste-farmaci-a-h. Accessed 1 Nov 2021
Reuken PA, Krnis K, Maaser C et al (2018) Microbial spectrum of intra-abdominal abscesses in perforating Crohn’s disease: results from a prospective German registry. JCC 697:701. https://doi.org/10.1093/ecco-JCC/JJY017
Hyder JA, Reznor G, Nakeam N et al (2016) Risk prediction accuracy differs for emergency versus elective cases in the ACS-NSQIP. Ann Surg 264:959–965
Mangram AJ, Horan TC, Pearson ML et al (1999) Guideline for prevention of surgical site infection 1999. Am J Infect Control 27:97–134
Bhakta A, Tafen M, Glotzer O et al (2016) Increased incidence of surgical site infection in IBD patients. Dis Colon Rectum 59:316–322
Nguyen GC, Leung W, Weizman AV (2011) Increased risk of vancomycin-resistant Enterococcus (VRE) infection among patients hospitalized for Inflammatory Bowel Disease in the United States. Inflam Bowel Dis 17:1338–1342
Mullen JT, Moorman DW, Davenport DL (2009) The obesity paradox body mass index and outcomes in patients undergoing non bariatric general surgery. Ann Surg 250:166–172
Di Carlo V, Gianotti L, Balzano G et al (1999) Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surg 16:320–326
Bratzler DN, Dellinger EP, Olsen KM et al (2013) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 14:73–156
Lantenbach E, Patel JB, Bilker WB et al (2001) Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumonia: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis 32:1162–1171
JD Siegel, E Rhinehart, M Jackson, L Chiarello, The HICPA Committee (2007) Guidelines for isolation precautions: preventing transmission of infections agents in healthcare settings. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html
Teillant A, Gandra S, Barter D et al (2015) Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis 15:1429–1437
Owens CD, Stoessel K (2008) Surgical site infections: epidemiology, microbiology and prevention. J Hosp Infect 70:3–10
Funding
Open access funding provided by University of Milan within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
DF: conception and design, surgical treatment, analysis and interpretation. AY: data acquisition. FC: data acquisition. GL: data acquisition. FC: surgical treatment, data acquisition. SR: microbiological expertise, data acquisition and interpretation. SA: infectious disease expertise, data interpretation. GMS: conception and design, surgical treatment, analysis and interpretation.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts to declare.
Research involving human participants and/or animals and Informed consent
The study was submitted to and approved by the Ethic Committee of the L. Sacco Hospital (#00-28004), and an informed consent was obtained from all the patients. The study was conducted according to the ethical standards of the Declaration of Helsinki (2013 version) and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foschi, D., Yakushkina, A., Cammarata, F. et al. Surgical site infections caused by multi-drug resistant organisms: a case–control study in general surgery. Updates Surg 74, 1763–1771 (2022). https://doi.org/10.1007/s13304-022-01243-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-022-01243-3