Abstract
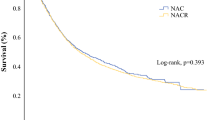
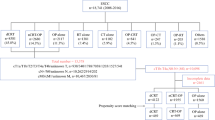
The optimal management of patients with locally advanced esophageal cancer remains under debate. We aimed to compare the long-term survival outcomes between definitive chemoradiation (dCR) and chemoradiation plus surgery (CRS) in patients with stage III esophageal adenocarcinoma (EAC). Using the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program registry, adults (≥ 18 years old) with diagnosis of AJCC 6th edition stage III EAC (T3/N1, T4/N0, and T4/N1) between 2004 and 2014 were included. A multivariable Cox regression was used to assess the effect of dCR and CRS on mortality. Of the 2633 patients included, 1115 (42%) underwent Dcr, and 1518 (58%) underwent CRS. The 5-year survival rate was 13% for patients undergoing dCR and 27% for patients undergoing CRS (p < 0.0001). Our observational data suggest that patients with stage III EAC may benefit by the use of esophagectomy after chemoradiotherapy.

Similar content being viewed by others
References
Hur C, Miller M, Kong CY et al (2013) Trends in esophageal adenocarcinoma incidence and mortality. Cancer 119(6):1149–1158
Arnold M, Laversanne M, Brown LM et al (2017) Predicting the future burden of esophageal cancer by histological subtype: international trends in incidence up to 2030. Am J Gastroenterol 112(8):1247–1255
National Cancer Institute Surveillance, Epidemiology, and End Results Program (2017) Cancer stats facts: esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed 27 Dec 2017
Stahl M, Budach W, Meyer HJ, Cervantes A, ESMO Guidelines Working Group (2010) Esophageal cancer: clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5):v46–v49
Hernan MA, Sauer BC, Hernandez-Diaz S et al (2016) Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 79:70–75
Stahl M, Stuschke M, Lehmann N et al (2005) Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23(10):2310–2317
Bedenne L, Michel P, Bouché O et al (2007) Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25(10):1160–1168
Vellayappan BA, Soon YY, Ku GY et al (2017) Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer. Cochrane Database Syst Rev 8:CD010511
Shao MS, Wong AT, Schwartz D et al (2016) Definitive or preoperative chemoradiation therapy for esophageal cancer: patterns of care and survival outcomes. Ann Thorac Surg 101(6):2148–2154
Sepesi B, Schmidt HE, Lada M et al (2016) Survival in patients with esophageal adenocarcinoma undergoing trimodality therapy is independent of regional lymph node location. Ann Thorac Surg 101(3):1075–1080
Wood MD, Zaki BI, Gordon SR et al (2013) Trimodality therapy for stage II–III carcinoma of the esophagus: a dose-ranging study of concurrent capecitabine, docetaxel, and thoracic radiotherapy. J Thorac Oncol 8(4):487–494
Funding
No funding was received for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was IRB exempted for using de-identified data of a national database.
Conflict of interest
The authors declare no financial or non-financial conflicts of interests.
Research involving human participants and/or animals
This study does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Rights and permissions
About this article
Cite this article
Schlottmann, F., Strassle, P.D., Gaber, C. et al. Stage III esophageal adenocarcinoma: definitive chemoradiation vs. chemoradiation plus surgery. Updates Surg 70, 423–426 (2018). https://doi.org/10.1007/s13304-018-0541-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-018-0541-5