Abstract
Introduction
Semaglutide, a glucagon-like peptide 1 receptor agonist (GLP1RA), is available in both parenteral and oral preparations. Studies of injectable preparations have convincingly demonstrated its beneficial effect on major adverse cardiac events (MACE). This predictive analysis was undertaken to forecast early termination of the SOUL trial (oral semaglutide) as well as the primary events.
Methods
SOUL is a multicenter, double-blind, placebo-controlled randomized controlled trial (RCT) evaluating the reduction in MACE associated with oral semaglutide versus placebo in patients with type 2 diabetes (T2D) and cardiovascular (CV) disease. A sample of 9642 participants will be followed for 5 years and 5 months. A random-effects model meta-analysis, pooling hazard ratios from previous RCTs, was conducted using R software to inform the predictive model. The background CV event rates from the placebo arms of previous RCTs with semaglutide were matched with the pre-adjudicated assumptions of the SOUL trial to create the predictive model. The truncated trial duration, MACE, and its individual components in the intervention and placebo arms were estimated. The predicted difference between the two groups was estimated using the chi-squared test.
Results
A pooled analysis of 10,013 patients revealed a significant reduction in the number of MACEs associated with semaglutide (HR 0.79, 95% CI 0.69–0.91). Predictive analysis indicated that 1225 events would be achieved by 3.78 years, suggesting premature termination.
Conclusion
The mathematical model based on the meta-analysis predicts that the SOUL study on oral semaglutide will be terminated early, with oral semaglutide showing benefits in terms of MACE compared to placebo. If the SOUL study corroborates the findings of this model, it may not only form the basis for the calculation of power but also define the duration of such studies, reducing costs and easing the process of designing cardiovascular outcome trials (CVOTs).
Protocol Registration
INPLASY202460061.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Current understanding states that CVOTs are crucial for assessing cardiovascular efficacy of diabetes treatments |
The research challenge is that many CVOTs end early because of less precise background cardiovascular risk estimation |
Using placebo groups from past RCTs, we improved the accuracy of background cardiovascular risk estimation |
Modified background risk for the SOUL trial predicts early termination at 3.78 years and a significant reduction in MACE compared to placebo through predictive modeling |
This strategy offers a more accurate and cost-effective blueprint for conducting future cardiovascular outcome trials |
Introduction
Type 2 diabetes (T2D) is one of the most common noncommunicable diseases and is strongly associated with cardiovascular disease and death [1]. It is also one of the most common causes of end-stage renal disease [2]. Treatment with RAAS blockade, statins, anti-platelets, and SGLT2 inhibitors is recommended by all major associations to reduce major adverse cardiovascular and renal outcomes, and these drugs are considered the standard of care [3]. Although renal and cardiac outcomes have improved over time with these medications, a therapeutic gap persists; renal and cardiovascular outcomes remain suboptimal in patients with T2D.
The LEADER trial of liraglutide, conducted on 9340 patients, was the first trial in which a GLP1RA was used to document an improvement in MACE in patients with T2D and established ASCVD [4]. Since then, other trials of multiple other GLP1RAs have demonstrated similar improvements in MACE and a probable improvement in renal outcomes [5].
Semaglutide, the newest GLP1RA, is widely used as the most powerful and efficacious GLP1RA [6]. The SUSTAIN 6 trial, conducted on 3297 patients and followed up for 104 weeks, compared once weekly injections of semaglutide with placebo in patients with T2D and established cardiovascular disease (previous cardiovascular, cerebrovascular or peripheral vascular disease), chronic heart failure (New York Heart Association class II or III) or chronic kidney disease ≥ stage 3 or age ≥ 60 years with at least one cardiovascular risk factor [7]. The primary outcome occurred in 6.6% of patients in the semaglutide group and in 8.9% of patients in the placebo group (hazard ratio, 0.74; 95% confidence interval [CI], 0.58–0.95; P < 0.001 for noninferiority). This difference was driven primarily by a reduction in nonfatal stroke, which occurred in 1.6% of patients in the semaglutide arm compared to 2.7% in the placebo group (hazard ratio, 0.61; 95% CI 0.38–0.99; P = 0.04). The rates of new or worsening nephropathy were lower in the semaglutide group. The recently published FLOW trial, conducted on 3533 patients and followed up for 177 weeks, comparing injectable once weekly semaglutide with placebo, revealed that semaglutide reduced the risk of clinically important kidney outcomes and death from cardiovascular causes in patients with type 2 diabetes and chronic kidney disease [8].
Oral semaglutide, the first and only orally available GLP1RA, is considered a major scientific breakthrough because of its unique mechanism and mode of action [9]. Naturally, the oral route makes it more attractive than its parenteral counterparts. The PIONEER trial program has studied the effects of oral semaglutide in patients with T2D in detail [10]. PIONEER 6 was conducted on 3183 patients and followed up for 69 weeks to assess the cardiovascular outcomes of once daily oral semaglutide in an event-driven, randomized, double-blind, placebo-controlled trial involving patients at high cardiovascular risk (aged ≥ 50 years with established cardiovascular or chronic kidney disease or aged ≥ 60 years with cardiovascular risk factors only) and revealed that oral semaglutide was safe in patients with T2D [11].
However, proof of its positive effect on cardiovascular and renal outcomes in patients with T2D remains elusive. The SOUL trial aims to provide this evidence [12].
In anticipation of this publication, a mathematical model was developed, and the results of the SOUL trial are predicted by this study. The search was conducted using the PICO search strategy [13].
P = Type 2 diabetes patients with high cardiovascular risk.
I = Semaglutide.
C = Placebo.
O = Reduction in composite CV events (MACE) and their individual components. In addition, a prediction analysis will be performed to predict the effective duration and outcome benefits in the ongoing SOUL study, aiming to suggest a means of reducing the cost of studies being designed for the future.
Methods
Study Design and Participants
The Semaglutide cardiOvascular oUtcomes (SOUL) trial is a multicenter, double-blind, placebo-controlled, randomized controlled trial (RCT). The primary aim of this study was to evaluate the reduction in major adverse cardiac events (MACE) associated with oral semaglutide compared with placebo in patients with type 2 diabetes (T2D) and established cardiovascular disease. Based on a 90% power calculation and a type 1 error of 0.025, the sample size was estimated to be 9642 participants. The plan is to follow the patients for 5 years and 5 weeks to target the first 1225 MACE [12]. This meta-analysis was conducted according to the recommendations of the PRISMA statement and registered with the International Platform of Registered.
Systematic Review and Meta-Analysis Protocols (INPLASY) on 17 June 2024 with INPLASY registration number INPLASY202460061 [14].
Systematic Literature Search
A comprehensive literature search was conducted in the Cochrane Library database to identify relevant RCTs with semaglutide and cardiovascular outcomes as the primary endpoints. The key search terms included “Type 2 diabetes” [MeSH], “Semaglutide,” and “Cardiovascular outcomes trial.” The date of publication was not limited. However, only articles published in English were selected. Additionally, manual searches for relevant citations were conducted using the Google Scholar search engine. The search strategy followed the PRISMA guidelines.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Study Selection and Data Extraction
The preliminary search yielded 2556 citations. After removing duplicates and using trials as a filter, 145 records were available for additional selection. After removing articles lacking full text, articles that were not relevant to the aim, and those that did not conform to the predefined inclusion criteria, three articles remained for analysis (Fig. 1). The data were extracted by two authors (SG and BS), and eligibility based on the predetermined inclusion criteria was assessed by both. Any discrepancy was addressed based on consensus.
The following predetermined inclusion criteria were used:
-
Age ≥ 18 years.
-
Patients with T2D.
-
The intervention arm included semaglutide.
-
Placebo was used as the comparator arm.
-
MACE was used as the primary endpoint.
-
The minimum duration of follow-up was 12 months.
Risk of Bias Assessment
The Cochrane risk of bias tool was used to assess the quality of the articles included in the analysis (Supplementary appendix A). The assessment was performed independently by both the authors, and disagreements were resolved through consensus.
Meta-Analysis of Prior Trials
To develop a robust predictive model for the SOUL trial, historical data on semaglutide and its impact on MACE are needed. These data were obtained by performing a meta-analysis of hazard ratios (HRs) from three previous RCTs (SUSTAIN 6, PIONEER 6, and FLOW). The relevant HRs and their 95% confidence intervals (95% CIs) were extracted from these studies. The ‘meta’ package in R was used to estimate the pooled HR.
Development of the Predictive Model
The predictive model for the SOUL trial was developed using the pooled HR estimate along with the preadjudicated assumptions mentioned in the trial protocol.
These assumptions include the following:
-
The annualized placebo MACE rate was 3.5%.
-
The true HR for the treatment effect was 0.83.
-
Uniform recruitment was allowed for 18 months.
-
The annual loss to follow-up rate was 1%.
-
The durations of the trials were 5 years and 5 weeks.
Effective Sample Size Estimation
The effective sample size was calculated using the following formula:
Effective sample size = N × (1 − annual loss to follow-up) total years, where N is the number of participants, and total years indicate the trial duration.
Event Rate Estimation
The following steps were used to calculate the event rates in the intervention and placebo groups:
-
The effective sample size was calculated for 1 year.
-
The annual number of events in the placebo group was estimated based on the placebo MACE rate.
-
The annual number of events in the intervention group was estimated based on the pooled HR from the meta-analysis.
-
The total annual number of events was calculated by summing events from both abovementioned groups.
Premature Termination Analysis
Because the desired number of events could be achieved well before the 5 years and 5 weeks of the trial had been completed, we calculated the time needed to reach the predetermined number of 1225 events using the following strategy:
-
The total number of events per year was estimated.
-
The total number of events was divided by the annual event rate to determine the estimated duration required to reach 1225 events.
Statistical Analysis
Statistical analysis was conducted using R software (R version 4.2.3 (2023-03-15)). The ‘metagen’ function from the ‘meta’ package was used to perform the meta-analysis, and the results are reported as logHRs (log-transformed HRs) and standard errors (SEs). The predictability and accuracy of the predictive model were assessed using a sensitivity analysis, varying key factors such as annual loss to follow-up and the placebo MACE rate. The statistical significance of the difference between the intervention and placebo groups was analyzed using the chi-square test. The dataset and codes used to conduct the analyses can be obtained from the corresponding author upon reasonable request.
Results
Baseline Characteristics of the Studies
This study analyzed 10,013 patients from three CVOTs and compared the benefit of semaglutide to that of a placebo in patients with T2D and established CVD or high CV risk. The semaglutide group included 5006 patients, and the placebo group included 5007 patients. The mean age ranged between 64.7 ± 7.1 and 66.6 ± 9.0 years, and the cohort was predominantly male. The patients in this analysis had a long duration of diabetes, with a mean baseline weight ranging between 89.5 ± 19.8 and 87 ± 1.5 kg, a mean HbA1c ranging between 7.8 ± 1.3% (61.7 ± 14.2 mmol/mol) and 8.7 ± 1.5% (70.9 ± 16.4 mmol/mol), and a duration of follow-up ranging between 69 and 177 weeks (Table 1). The quality of the individual studies was assessed using the Cochrane risk of bias algorithm, and publication bias was assessed using funnel plot asymmetry. The latter was assessed qualitatively when the number of studies was < 10 (Supplementary Appendix B).
Pooled Meta-Analysis
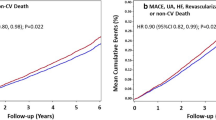
The pooled analysis showed a significant reduction in the primary endpoint (MACE), with an HR of 0.79 (95% CI 0.69–0.91). The primary endpoint was predominantly driven by a reduction in CV death (HR 0.73, 95% CI 0.54–0.99) (Fig. 2).
Development of the Predictive Model
In addition to the pooled HR from the meta-analysis, estimation of the annual event rates was the principal component needed to construct the model. The following formula was used to calculate the effective annualized sample size:
The expected annual numbers of events in the intervention and placebo groups were estimated to be 147 and 177, respectively. The total annual expected number of events was 324.
Predictive Model for SOUL
Based on the duration of the study mentioned in the protocol (5 years and 5 months) as well as the predetermined assumptions, the SOUL study was estimated to accumulate a total number of 1464 events, with 817 events in the semaglutide group and 647 in the placebo group. The chi-squared test was used to determine whether this difference in events between the two groups was statistically significant. The chi-squared statistic was 19.74 (df = 1), with a df value for significance < 0.001.
Premature Termination Analysis
Because most RCTs terminate trials once the desired number of events has been achieved, we analyzed the duration needed to reach 1225 events. Based on the annualized event rates in the semaglutide and placebo groups, the SOUL trial was calculated to accumulate 1225 events at the end of 3.78 years, resulting in premature termination (Fig. 3). The predicted numbers of MACE events in the semaglutide and placebo groups were 578 and 647, respectively. The chi-squared test (3.88, df = 1) indicated a significant difference between the two groups (p = 0.04).
Sensitivity Analysis
Sensitivity analyses were conducted to evaluate the robustness of the predictive model by varying key parameters, such as the annual loss to follow-up rate and the placebo MACE rate. The results of these analyses confirmed the stability of the model, with the estimated duration to reach 1225 events remaining within a reasonable range under different scenarios.
Subgroup Predictive Analysis
The predicted number of events for NFMI, NFS, and CV death required additional calculation to obtain a historical baseline in the placebo arm for each of these variables. This calculation was based on the total number of MACE events reported in the three studies and using the proportion of each on the predicted MACE (1225 events). The predicted numbers of events are detailed in Table 2.
A chi-squared test was performed to identify whether the difference between the two groups was statistically significant for each of the individual components. The difference was not significant for NFMI (χ2 2.22, df 1, p 0.14) or NFS (χ2 1.78, df 1, p 0.18). However, the difference was significant for the CV death endpoint (χ2 13.66, df 1, p 0.0002).
Discussion
Individual patient-level data combined with SUSTAIN 6 (once weekly injectable semaglutide versus placebo) and PIONEER 6 (oral semaglutide versus placebo) were collected to assess MACE and HF in patients with and without established CV disease and/or chronic kidney disease, prior MI or stroke, and prior HF. This post hoc analysis of both studies revealed that semaglutide had consistent effects on MACE across varying CV risk groups. This benefit was driven mainly by a significant reduction in all-cause stroke caused by semaglutide. No effect of semaglutide on MACE was observed in subjects with prior HF [15].
A pooled analysis of these data also revealed that semaglutide significantly slowed the rate of kidney function decline, irrespective of kidney function at baseline [16]. This finding has been convincingly reiterated by FLOW, a randomized controlled trial that has shown significant improvement in renal and cardiovascular outcomes in patients with established CKD [8].
This meta-analysis of all CVOTs conducted with oral and parenteral semaglutide to date revealed that the use of semaglutide as a molecule resulted in a significant 21% reduction in MACE, which was driven by reduction in CV death (HR 0.79, 95% CI 0.61–0.90).
In the past, several trials were terminated prematurely because the required number of events was accrued earlier than the estimated duration. These studies include the CARMELINA, CAROLINA, CREDENCE, EMPAREG, DECLARE TIMI-58, LEADER, SUSTAIN 6, PIONEER 6, REWIND, EXSCEL, ELIXA, DAPA-CKD, and EMPA-kidney trials [7, 11, 22–27]. The estimated duration of these studies and the required events were based on the CV risk in the population determined by published prevalence or cross-sectional studies. While earlier trials did not have dedicated RCTs at their disposal, studies such as the SOUL trial had 3 prior RCTs to refer to, as far as baseline CV risk estimation was concerned. In fact, both PIONEER 6 and SUSTAIN 6 were prematurely terminated, reflecting a lack of accurate estimation of the time needed to attain the required number of CV events.
With the availability of data from three CVOTs with semaglutide, this meta-analysis provides a platform for designing a mathematical model to predict the outcomes of the ongoing SOUL trial, a CVOT comparing oral semaglutide with placebo.
Because the placebo arms in all the CVOTs now reflect the background CV risk in the population, the study duration required to obtain the desired number of events can be estimated far more accurately based on power calculations.
The calculated duration of the SOUL trial was 5 years and 5 weeks based on a baseline annualized CV risk of 3.5%. However, using the background MACE rate in the population in the placebo arm of the PIONEER 6, SUSTAIN 6, and FLOW trials, the duration required to reach 1225 events can be accurately estimated to be 3.78 years.
The duration required to obtain the desired number of events can be estimated from previous CVOTs, and the number of probable events in the placebo and intervention arms can both be estimated while predicting the direction of the effect size (hazard ratio). This analysis predicts that the required number of events (1225) will have occurred after 3.78 years. This analysis also predicted that the estimated number of events (MACE) in the semaglutide and placebo groups would be 647 and 578, respectively.
This difference in events was significant at 0.05, indicating that we can expect superiority from the primary end point of the SOUL trial.
The annualized MACE rate in the pooled placebo arm was 5.66% (per our calculation), in contrast to the 3.5% reported in the SOUL trial. This difference explains why the trial will likely be terminated early, and a more objective background risk estimation needs to be performed (if available) compared to heterogeneous prevalence studies.
This mathematical prediction will be subject to scrutiny once the original SOUL trial has been published. However, once it is proven to be accurate, this mathematical prediction may be used to calculate not only the power but also the duration of such studies. In addition to making such RCTs more practical, they will significantly reduce the cost of conducting such expensive projects, providing relief for both industry and researchers.
Limitations
The principal limitations are related to the fact that we analyzed means from the RCTs instead of individual patient-related data. The number of studies included in the present study was too low to conclusively assess heterogeneity. In addition, the annual CV event rate was assumed to be constant but may have been affected by the COVID-19 pandemic.
Strengths
The main strength of this analysis is related to the pooling of the placebo CV event rates from the three trials, providing us with a robust backdrop to compare the intervention-related outcomes. Traditionally, the background event rates in the population are based on cross-sectional or prevalence studies. In addition, the selection of a conservative random effect model would increase the credibility of the outcomes.
Conclusion
This meta-analysis clearly demonstrated that semaglutide reduces MACEs primarily by a reduction in CV-related deaths. More interestingly, we predict that the SOUL trial will be prematurely terminated after 3.78 years and that oral semaglutide will significantly reduce MACEs. If proven accurate, this model has great potential for improving structuring and, more importantly, reducing expenses while planning future CVOTs.
References
Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;15;5(4):444–70. https://doi.org/10.4239/wjd.v5.i4.444. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4127581/
Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end-stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015;4(2):28–33. https://doi.org/10.12861/jrip.2015.07. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4459725/
American Diabetes Association Professional Practice Committee; 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes—2024. Diabetes Care 2024; 47 (Supplement_1): S158–78. https://doi.org/10.2337/dc24-S009
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et.al. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827. https://pubmed.ncbi.nlm.nih.gov/27295427/
Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189. https://doi.org/10.1186/s12933-021-01366-8.
Dhillon S. Semaglutide: first global approval. Drugs. 2018;78:275–84. https://doi.org/10.1007/s40265-018-0871-0.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141. https://pubmed.ncbi.nlm.nih.gov/27633186/
Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024. https://doi.org/10.1056/NEJMoa2403347. (Accessed on: 6th June 2024).
Rasmussen MF. The development of oral semaglutide, an oral GLP-1 analog, for the treatment of type 2 diabetes. Diabetol Int. 2020;11(2):76–86. https://doi.org/10.1007/s13340-019-00423-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7082439/
Brunton SA, Mosenzon O, Wright EE Jr. Integrating oral semaglutide into clinical practice in primary care: for whom, when, and how? Postgrad Med. 2020;132(sup2):48–60. https://doi.org/10.1080/00325481.2020.1798162.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR et al. PIONEER 6 Investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. https://doi.org/10.1056/NEJMoa1901118. https://pubmed.ncbi.nlm.nih.gov/31185157/
McGuire DK, Busui RP, Deanfield J, Inzucchi SE, Mann JFE, Marx N et al. Effects of oral semaglutide on cardiovascular outcomes in individuals with type 2 diabetes and established atherosclerotic cardiovascular disease and/or chronic kidney disease: Design and baseline characteristics of SOUL, a randomized trial. Diabetes Obes Metab. 2023;25(7):1932–41. https://doi.org/10.1111/dom.15058. https://pubmed.ncbi.nlm.nih.gov/36945734/
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021, 29;372:n160. https://doi.org/10.1136/bmj.n160. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8005925/
The protocol for this systematic review was registered on INPLASY (ID: INPLASY202460061) and is available in full on inplasy.com.
Husain M, Bain SC, Jeppesen OK, Lingvay I, Sørrig R, Treppendahl MB et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22(3):442–51. https://doi.org/10.1111/dom.13955. https://pubmed.ncbi.nlm.nih.gov/31903692/
Tuttle KR, Bosch-Traberg H, Cherney DZI, Hadjadj S, Lawson J, Mosenzon O et al. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo. Kidney Int. 2023;103(4):772–81. https://doi.org/10.1016/j.kint.2022.12.028. https://pubmed.ncbi.nlm.nih.gov/36738891/
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N et al. Effect of Linagliptin vs Placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA Randomized Clinical Trial. JAMA. 2019;321(1):69–79. https://doi.org/10.1001/jama.2018.18269. https://jamanetwork.com/journals/jama/fullarticle/2714646
Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ et al. Effect of Linagliptin vs Glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA Randomized Clinical Trial. JAMA. 2019;322(12):1155–66. https://doi.org/10.1001/jama.2019.13772. https://jamanetwork.com/journals/jama/fullarticle/2751398
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM et al. CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. https://doi.org/10.1056/NEJMoa1811744. https://pubmed.ncbi.nlm.nih.gov/30990260/
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720. https://pubmed.ncbi.nlm.nih.gov/26378978/
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A et al. DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389. https://pubmed.ncbi.nlm.nih.gov/30415602/
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA et al. LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827. https://pubmed.ncbi.nlm.nih.gov/27295427/
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P et al. REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. https://doi.org/10.1016/S0140-6736(19)31149-3. https://pubmed.ncbi.nlm.nih.gov/31189511/
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB et al. EXSCEL Study Group. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. https://doi.org/10.1056/NEJMoa1612917. https://pubmed.ncbi.nlm.nih.gov/28910237/
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV et al. ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. https://doi.org/10.1056/NEJMoa1509225. https://pubmed.ncbi.nlm.nih.gov/26630143/
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE et al. DAPA-CKD trial committees and investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816. https://pubmed.ncbi.nlm.nih.gov/32970396/
The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117–27. https://doi.org/10.1056/NEJMoa2204233. https://pubmed.ncbi.nlm.nih.gov/36331190/
Acknowledgements
We acknowledge all the investigators and patients who participated in the trials included in this analysis.
Funding
No funding was obtained for this study. The rapid service fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Binayak Sinha conceptualized the study. Binayak Sinha conducted an expanded literature review to contextualize the results of the meta-analysis. The analysis was performed by Samit Ghosal Binayak Sinha, who cross-verified the search strategy as well as the quality of the publications. Binayak Sinha and Samit Ghosal contributed to the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Binayak Sinha and Samit Ghosal have nothing to declare.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sinha, B., Ghosal, S. Forecasting Trial Milestones: A Predictive Analysis for Early Termination of the SOUL Study. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01635-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01635-1