Abstract
Introduction
The triglyceride–glucose index (TyG) has been identified as a dependable and simple indicator marker of insulin resistance (IR). Research has demonstrated a correlation between macrovascular complications and TyG. However, limited research exists regarding the relationship between TyG and diabetic microvascular complications. Consequently, the objective of this study is to investigate the association between TyG and diabetic kidney disease (DKD) and diabetic peripheral neuropathy (DPN).
Methods
This is a cross-sectional, observational study. A total of 2048 patients from Tongren Hospital, Shanghai Jiao Tong University School of Medicine were enrolled. The primary outcomes are DKD and DPN. Quantile regression analysis was employed to investigate the implicit factors of TyG quartiles. Subsequently, based on implicit factors, logistic regression models were constructed to further examine the relationship between TyG and DKD and DPN.
Results
In the baseline, TyG exhibited higher values across patients with DKD, DPN, and co-existence of DKD and DPN (DKD + DPN) in type 2 diabetes (T2D). Univariate logistic regressions demonstrated a significant association between an elevated TyG and an increased risk of DKD (OR = 1.842, [95% CI] 1.317–2.578, P for trend < 0.01), DPN (OR = 1.516, [95% CI] 1.114–2.288, P for trend < 0.05), DKD + DPN (OR = 2.088, [95% CI] 1.429–3.052, P for trend < 0.05). Multivariable logistic regression models suggested a statistically significant increase in the risk of DKD (OR = 1.581, [95% CI] 1.031–2.424, p < 0.05), DKD + DPN (OR = 1.779, [95% CI] 1.091–2.903, p < 0.05) after adjusting the implicit factors of TyG quartiles. However, no significant relationship was observed between TyG and DPN in the multivariable regression analysis.
Conclusions
Elevated TyG was significantly associated with an increased risk of DKD in T2D, but no significant relationship was shown with DPN. This finding provided further evidence for the clinical significance of integrating TyG into the initial assessment of diabetic microvascular complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Studies have found that the triglyceride–glucose index (TyG) is linked to macrovascular damage, but there is limited research on its connection to diabetic microvascular complications. |
The objective of this study is to investigate the association between TyG and both diabetic kidney disease (DKD) and diabetic peripheral neuropathy (DPN). |
What was learned from the study? |
We found that elevated TyG was significantly associated with an increased risk of DKD in type 2 diabetes (T2D) but no significant relationship was shown with DPN. |
This study provides further evidence for the integration of TyG in the initial assessment of diabetic microvascular complications. |
Introduction
Diabetes is a chronic disease with a global prevalence. According to the International Diabetes Federation Diabetes Atlas 2021, there are currently 537 million adults living with diabetes worldwide [1]. Notably, China has the largest number of people with type 2 diabetes (T2D), with an estimated 140.9 million individuals affected [1]. Continuous hyperglycemia and insulin resistance (IR) could lead to vascular damage and a chain of metabolic disorders, ultimately impairing quality of life and significantly elevating the overall risk of premature mortality. Traditionally, complications of diabetes mellitus have been categorized into macrovascular and microvascular complications. In a recent observational study conducted in 28 countries, it was found that microvascular complications were present in 50% of patients with T2D [2].
Diabetic kidney disease (DKD) is a common microcirculatory disorder in diabetes, which has surpassed other complications to become the first leading driver of incident kidney failure [3]. Patients with DKD are not only at risk for future kidney failure but also have a significantly increased risk of developing cardiovascular disease [4]. Risk-prediction models demonstrate that hyperglycemia, elevated blood pressure, dyslipidemia, and IR are the most important risk factors for DKD [5]. Meanwhile, diabetic peripheral neuropathy (DPN), as one of the most common chronic diabetic complications, increases the risk of ulceration non-invasive amputation, foot infection, and eventually leads to long-term disability [6, 7]. Evidence has shown that the incidence of DPN is associated with age, obesity, and elevated blood glucose and triglycerides [8,9,10]. In a study of patients with T2D, there was a graded relationship between triglyceride levels and the risk of lower-limb amputations [11]. Finding a common and straightforward indicator to predict the occurrence of both DKD and DPN presents a considerable challenge due to their multifactorial nature and the involvement of various risk factors. However, it is worth exploring potential indicators that may provide valuable insights into the risk of developing both complications.
Recent studies have shown promising results in using the triglyceride–glucose index (TyG) as a potential marker for IR and associated complications [12,13,14,15,16]. TyG, calculated as ln[fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2], has been proven as a reliable and simple substitute marker of IR [17, 18]. Several previous studies indicated that TyG is associated with coronary artery calcification (CAC) and cardiovascular disease (CVD) in T2D [12, 13]. Studies in a Chinese population showed that elevated TyG is independently correlated with an increased risk of incident diabetes and arterial stiffness and renal microvascular injury [14]. Chronic hyperglycemia and IR are known to contribute to vascular injury through various pathophysiological processes [15]. Monitoring TyG as a surrogate marker of IR may be crucial for the prevention and treatment of T2D and its complications.
Research has shown that in hospitalized patients with T2D, an elevated TyG index is associated with a high risk of lower limb stenosis and renal microvascular injury [16]. These findings indicate the potential utility of TyG in assessing the risk of DKD and DPN in individuals with T2D. Further research is needed to validate the TyG as a predictive tool for DKD and DPN and to understand its underlying relations.
In this study, we assemble a large group of patients with T2D to examine the association between TyG and both DKD and DPN. The results of our study may provide further evidence for the integration of TyG in the initial assessment of diabetic microvascular complications.
Methods
Participants
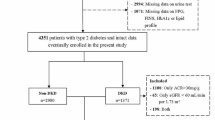
We conducted a cross-sectional, observational study and a total of 2048 patients were enrolled. All patients were diagnosed with T2D and admitted to the Department of Endocrinology, Tongren Hospital, Shanghai Jiao Tong University School of Medicine from May 1, 2020 to February 28, 2023. Exclusion criteria were as follows: (1) age less than 30 or greater than 75 years; (2) had a diagnosis of urinary tract infection, renal calculi, or primary renal disease, estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2; (3) lack of urinary albumin/creatinine ratio (UACR), vibration perception threshold (VPT) data. Finally, 1931 patients were enrolled and included: 409 patients with diabetic kidney disease (DKD), 327 patients with diabetic peripheral neuropathy (DPN), and 255 patients with co-existence of DKD and DPN (DKD + DPN) (Fig. 1).
Ethical Approval
All participants were carefully informed about the protocol and provided written informed consent before inclusion in the study. All study procedures were performed in accordance with Helsinki Declaration of 1964 and its later amendments. This study protocol was reviewed and approved by the Ethics Committee of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (K2021-003-01).
Index Measurements Method
In this study, baseline information regarding demographic characteristics, medication history, diagnosis history of diseases, smoking history, and alcohol consumption history were extracted from the inpatient medical record system of Tongren Hospital, Shanghai Jiao Tong University School of Medicine. Anthropometric data including weight, height, body mass index (BMI), waist circumference (WC), and blood pressure (BP) were collected using standardized measurement techniques. BP measurements were obtained through the use of an automatic electronic blood pressure monitor after resting for ≥ 5 min (model high blood pressure (HBP)-9021; Omron Corp, Kyoto, Japan). BMI was calculated based on weight and height, while WC was measured with the participant in the standing position. Furthermore, various biochemical parameters were assessed using fasting venous blood samples. These parameters included fasting c-peptide (FCP), glycosylated hemoglobin A1c (HbA1c), fasting blood glucose (FBG), triglycerides (TG), alanine aminotransferase (ALT), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and uric creatinine (Cr). The urinary albumin/creatinine ratio (UACR) was calculated based on first-morning urine samples. Additionally, TyG was calculated as: ln [fasting triglycerides (mg/dl) × fasting glucose (mg/dl)/2].
Assessments of DKD and DPN
DKD is characterized by impaired kidney function in individuals with diabetes, after excluding other causes of chronic kidney disease. According to the Kidney Disease: Improving Global Outcomes (KDIGO), the diagnosis of DKD is based upon the findings of decreased estimated glomerular filtration rate (eGFR < 60 ml/min/1.73 m2) and/or increased urinary albumin excretion (≥ 30 mg/g creatinine) persisting for > 3 months [19]. DPN is evaluated using the Diabetes Neuropathy Symptom Score (DNS) and Vibration Perception Threshold (VPT) [20, 21]. The symptoms of DNS encompass numbness, tingling, pain, and unsteadiness in a “stocking and glove” distribution, each symptom was assigned a scored of one point and a total score of ≥ 1 was considered indicative of abnormality. The VPT assessment was performed using the biothesiometer by the same operator, adhering to the guidelines established by the International Working Group on the Diabetic Foot of the International Diabetes Federation. DPN was defined as VPT > 25 V.
Statistical Analysis
Statistical analysis was performed using SPSS statistics software (V.26). Normally distributed measurements were described by the mean and SD (x ± s); for non-normally distributed measurements, the median (interquartile ranges, IQR) were used. Categorical variables were presented as frequencies and percentages. The Wilcoxon rank-sum test was employed to compare quantitative variables at baseline, while the chi-squared test was used for categorical data. TyG was grouped by quartiles when analyzing the association between TyG and DKD, DPN and DKD + DPN in univariate logistic regression. Quantile regression analysis was employed to investigate the association between TyG quartiles and diabetic risk factors. Furthermore, multivariate logistic regression models were constructed to assess the association between TyG quartiles and DKD, DPN, and DKD + DPN, taking into account various factors. Model 1 adjusted for sex and gender. Model 2, built upon Model 1, included the non-traumatization indicator: systolic blood pressure (SBP). Model 3 encompassed complete adjustments for laboratory markers, including HbA1c, TC, HDLc, and LDLc. Finally, Model 4 incorporated smoking and drinking history. P < 0.05 is considered significant.
Results
The characteristics of the participants are shown in Table 1. In this study, a total of 1931 hospitalized patients with T2D were enrolled, consisting of 1185 (61.4%) men and 746 (38.6%) women, with an average age of 60 years (ranging from 48 to 66 years). The average duration of diabetes of the participants was 5.25 years (ranging from 0.25 to 12.41 years). Compared to the no DKD or DPN (NDKD + NDPN) group, the DKD group had a longer duration of diabetes and higher levels of SBP, BMI, WC, TG, Cr, and UACR. The DPN group had a lower age and H-LDLc, but a longer duration of diabetes and higher BMI, WC, HbA1c, and UACR. Moreover, TyG was higher in all three groups: the DKD group, DPN group, and DKD + DPN group. These findings indicate that both DKD and DPN are associated with metabolic disorders such as higher BMI, WC, and TyG, which may be potential risk factors for the development of these complications in patients with T2D.
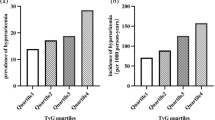
According to the quartiles of their TyG index levels (Table 2), the patients were divided into four groups, Q1 (4.80 ≤ TyG index ≤ 6.98), Q2 (6.99 ≤ TyG index ≤ 7.47), Q3 (7.48 ≤ TyG index ≤ 8.03) and Q4 (8.04 ≤ TyG index ≤ 11.02). Univariate logistic regression analyses were conducted with Q1 as the reference group. The results showed that higher TyG index levels in Q4 were associated with an increased risk of DKD, DPN, and DKD + DPN. Specifically, compared to Q1, TyG levels in Q4 were associated with an increased risk of DKD (OR = 1.842, [95% CI] 1.317–2.578, P for trend < 0.01), DPN (OR = 1.516, [95% CI] 1.114–2.288, P for trend < 0.05), DKD+DPN (OR = 1.516, [95% CI] 1.114–2.288, P for trend < 0.05).
The quantile regression was used to explore the association between TyG quartiles and diabetic risk factors. As shown in Table 3, age, SBP, FCP, HbA1C, ALT, TC, HDLc, LDLc, and drinking history are the implicit factors of TyG quartiles (p < 0.05). Based on the implicit factor of TyG quartile, we performed four models in multivariate logistic regressions to further verify the relationship between TyG quartile and DKD, DPN, and DKD + DPN. Taking Q1 as reference, multivariable logistic regression analysis revealed that TyG levels for Q2, Q3, and Q4 increased the odds ratios for DKD (Table 4). In Model 4 (adjusted for sex, age, SBP, HbA1c, TC, HDLc, LDLc, drinking and smoking history), risks in the Q4 group of DKD (OR = 1.581, [95% CI] 1.031–2.424) and DKD&DPN (OR = 1.779, [95% CI] 1.091–2.903) were still significantly higher than they were in the Q1 group (p < 0.05). This further proved that an increased TyG would lead to DKD. However, no significant relationship was observed between TyG and DPN in the four models.
Discussion
The objective of our study was to investigate the link between TyG, a marker of IR, and the risk of microvascular complications (including DKD and DPN) in patients with T2D. Employing logistic regression models, we identified a graded relationship between TyG and the prevalence of DKD. Nevertheless, further prospective and multi-center studies are required to confirm the association between TyG and DPN.
In recent years, the prevalence of diabetes has risen continuously, resulting in an epidemic of diabetes complications. The meta-analysis conducted in China revealed that 21.8% of patients with diabetes have concomitant DKD [22]. Additionally, two population-based studies utilizing door-to-door screening reported prevalence estimates of 1–4% for neuropathy, with 40–55% of cases found to be secondary to diabetes [23, 24]. In this study, the prevalence rates of DKD and DPN were 21.18% and 16.93%, respectively. The prevalence rate of DKD + DPN was 13.21%. Even after adjusting for potential confounding factors, the results showed a strong association between TyG and DKD (OR = 1.581, [95% CI] 1.031–2.424). The findings suggest that TyG may serve as an effective indicator for identifying DKD in patients with T2D. However, no significant relationship was shown with DPN.
Patients with T2D may require hospitalization for various reasons beyond metabolic issues. In our study, participants were hospitalized primarily for metabolic reasons, including poor glycemic control or the onset or worsening of diabetic complications. Consequently, the association analyses conducted are less susceptible to the influence of concurrent acute conditions. Overall, noticeable differences in age, SBP, BMI, WC, HbA1c, FBG, lipid metabolism, and TyG were observed in DKD and DPN group compared with patients with NDKD + NDPN group. These findings are consistent with previous research [5, 8,9,10]. Through the effective management and control of risk factors such as hyperglycemia, elevated blood pressure, obesity, and dyslipidemia, it is plausible to decelerate the progression of diabetic complications and avert the onset of DKD and DPN [25, 26]. However, these baseline data also underscore the significance of the observed disparities in TyG, warranting serious consideration.
TyG, a newly proposed indicator in recent years, has been shown to be a reliable and simple substitute marker of IR [17, 18]. Our results revealed that elevated FBG and TG have been associated with the occurrence of DKD and DPN. Hence, TyG, which comprises FBG and TG, also exhibited differences between the three group in the baseline. In univariate logistic regressions, an elevated TyG index was found to be associated with a higher risk of DKD. However, since TyG is an indicator of metabolism composed of FBG and TG, it can be influenced by various metabolic conditions. Therefore, through quantile regression, we also identified several implicit factors associated with TyG quartiles, including sex, age, SBP, HbA1c, FCP, TC, HDL-C, LDL-C, and drinking habits. These indicators are primarily related to metabolism and can provide further insights into the association between TyG and microvascular complications in patients with T2D. After controlling for confounders, we still observed a significant increase in the risk of diabetic kidney disease DKD.
The original emergence of TyG was for finding an available measure to replace the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index [17]. IR has been found to be independently associated with DKD [27], extending beyond its indirect connections with glucose levels, blood pressure, body weight, and lipid control. Numerous studies have consistently demonstrated a significant association between a higher TyG index and various conditions, including cardiovascular disease (CVD), obesity, diabetes, non-alcoholic fatty liver disease, and kidney disease [12,13,14,15,16, 28, 29]. As a predictive tool for the occurrence of DKD, TyG reflects not only blood glucose levels and lipid levels but also IR and obesity. It should be noted that TyG is not a single indicator but rather a multifactorial index that takes into account multiple parameters.
Although our multivariable logistic regression analysis did not reveal a significant relationship between TyG and DPN, we maintain the belief that TyG could potentially play a crucial role as a risk factor for DPN. Previous studies have demonstrated a close correlation between DPN and the duration of diabetes as well as HbA1c levels [30]. Furthermore, independent of HbA1c levels, the presence of multiple metabolic syndrome components, such as hypertriglyceridemia, hypertension, abdominal obesity, and low HDL levels, consistently exhibit an association with DPN in patients with T2D [31, 32]. It is worth noting that dyslipidemia is highly prevalent in individuals with T2D [33] and has been linked to the development of DPN [34]. A study involving 200 patients with T2D demonstrated a significant correlation between TC levels exceeding 5.2 mmol/l and the presence of DPN [35]. In our study, the average TG level in the DPN group was normal. Additionally, 1108 participants (57.4%) had received statin therapy, with 540 (57.4%) in the NDKD + NDPN group, 173 (52.7%) in the DPN group, 237 (57.9%) in the DKD group, and 156 (62%) in the DKD + DPN group. There was no statistically significant difference in statin therapy compared to the NDKD + NDPN group. Therefore, considering multiple metabolic factors is essential when evaluating the risk of DPN in individuals with T2D, as each factor could contribute to the occurrence and progression of this complication.
Study Limitations
Although we attempted to find the implicit factors of TyG to improve the accuracy of the results, there were still some limitations in our study. First, there is a selection bias in the population studied because the patients with T2D were collected from the inpatient department of Shanghai Tongren Hospital. The sample size of the data obtained is limited, and the data have a certain region, which is not representative. Second, the study included only 30 to 75-year-old Chinese participants, so we should be cautious in extrapolating the present findings to other subjects. Third, TyG, as an indicator of metabolism, can be affected by some metabolic conditions, such as primary triglyceride abnormalities.
Furthermore, measurements of the TyG index in a hospital setting may be influenced by the administration of hypoglycemic agents and lipid-lowering medications, potentially confounding the results. Therefore the TyG has high sensitivity, but low specificity, for screening the onset of diabetic microvascular complications.
Conclusions
Elevated TyG was significantly associated with an increased risk of DKD in T2D, but no significant relationship was shown with DPN. This finding provided further evidence for the clinical significance of integrating TyG into the initial assessment of diabetic microvascular complications.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
International Diabetes Federation. IDF Diabetes Atlas, 2021, https://www.diabetesatlas.org.
Litwak L, Goh SY, Hussein Z, Malek R, Prusty V, Khamseh ME. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol Metab Syndr. 2013;5(1):57.
Koye DN, Magliano DJ, Nelson RG, Pavkov ME. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis. 2018;25(2):121–32.
Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273–80.
Jiang W, Wang J, Shen X, et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta-analysis of 20 cohorts. Diabetes Care. 2020;43(4):925–33.
Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–13.
Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the national health and nutrition examination survey. Otol Neurotol. 2010;31(9):1445–50.
Wu B, Niu Z, Hu F. Study on risk factors of peripheral neuropathy in type 2 diabetes mellitus and establishment of prediction model. Diabetes Metab J. 2021;45(4):526–38.
Andersen ST, Witte DR, Dalsgaard EM, et al. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-Denmark. Diabetes Care. 2018;41(5):1068–75.
Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397–405.
Callaghan BC, Feldman E, Liu J, et al. Triglycerides and amputation risk in patients with diabetes: ten-year follow-up in the DISTANCE study. Diabetes Care. 2011;34(3):635–40.
Park K, Ahn CW, Lee SB, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
Zhao S, Yu S, Chi C, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18(1):95.
Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275.
Pan Y, Zhong S, Zhou K, et al. Association between diabetes complications and the triglyceride-glucose index in hospitalized patients with type 2 diabetes. J Diabetes Res. 2021;2021:8757996.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–4335.
Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61(10):533–40.
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98 (4S):S1-S115.
Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the diabetic neuropathy symptom score. Diabet Med. 2002;19(11):962–5.
Garrow AP, Boulton AJ. Vibration perception threshold—a valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab Res Rev. 2006;22(5):411–9.
Zhang XX, Kong J, Yun K. Prevalence of diabetic nephropathy among patients with type 2 diabetes mellitus in China: a meta-analysis of observational studies. J Diabetes Res. 2020;2020:2315607.
Beghi E, Monticelli ML. Chronic symmetric symptomatic polyneuropathy in the elderly: a field screening investigation of risk factors for polyneuropathy in two Italian communities Italian General Practitioner Study Group (IGPST). J Clin Epidemiol 1998;51 (8):697–702
Bharucha NE, Bharucha AE, Bharucha EP. Prevalence of peripheral neuropathy in the Parsi community of Bombay. Neurology. 1991;41(8):1315–7.
Ueki K, Sasako T, Okazaki Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99(1):256–66.
Cernea S, Raz I. Management of diabetic neuropathy. Metabolism. 2021;123: 154867.
Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55(5):1456–62.
Li X, Li G, Cheng T, Liu J, Song G, Ma H. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study : TyG index and incident diabetes. Lipids Health Dis. 2020;19(1):236.
Xue Y, Xu J, Li M, Gao Y. Potential screening indicators for early diagnosis of NAFLD/MAFLD and liver fibrosis: triglyceride glucose index-related parameters. Front Endocrinol (Lausanne). 2022;13: 951689.
Shang J, Yu D, Cai Y, et al. The triglyceride glucose index can predict newly diagnosed biopsy-proven diabetic nephropathy in type 2 diabetes: a nested case control study. Medicine (Baltimore). 2019;98(46): e17995.
Tesfaye S, Chaturvedi N, Eaton SEM, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50.
Vinik AI, Nevoret ML, Casellini C, Parson H. Diabetic neuropathy. Endocrinol Metab Clin North Am. 2013;42(4):747–87.
Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–48.
Song L, Zhou L, Tang Z. An association analysis of lipid profile and diabetic cardiovascular autonomic neuropathy in a Chinese sample. Lipids Health Dis. 2016;15:122.
Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14(4):257–67.
Acknowledgements
We especially acknowledge all participants involved in this study. We thank all participants for their invaluable contribution to the study.
Funding
This study was supported by Changning District Committee of Science and Technology (CNKW2017Y06), Master and Doctor innovation talent base for endocrine and metabolic diseases (RCJD2021S03), Natural Science Foundation Project of Shanghai Scientific and technological innovation plan (No.22ZR1457000), Research Fund of Shanghai Tongren Hospital, Shanghai Jiaotong University School of Medicine (TRYJ2021LC07) and Rising Star Programme in Tongren Hospital (TRKYRC-xx202209). The journal’s Rapid Service Fee was funded by Master and Doctor innovation talent base for endocrine and metabolic diseases (RCJD2021S03).
Author information
Authors and Affiliations
Contributions
Shan Huang, Xiaohong Jiang, and Fan Hu designed the research; Juan Du and Xiaoxu Ge conducted the research; Zhihui Tu ran the statistical analyses and wrote the paper. Wenfang Peng, Lisha Shen, and Lili Xia assisted with data acquisition and interpretation. Shan Huang and Fan Hu made revisions and provided critique. All author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Zhihui Tu, Juan Du, Xiaoxu Ge, Wenfang Peng, Lisha Shen, Lili Xia, Xiaohong Jiang, Fan Hu, and Shan Huang have nothing to disclose.
Ethical Approval
All participants were carefully informed about the protocol and provided written informed consent before inclusion in the study. All study procedures were performed in accordance with Helsinki Declaration of 1964 and its later amendments. This study protocol was reviewed and approved by the Ethics Committee of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (K2021-003–01).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tu, Z., Du, J., Ge, X. et al. Triglyceride Glucose Index for the Detection of Diabetic Kidney Disease and Diabetic Peripheral Neuropathy in Hospitalized Patients with Type 2 Diabetes. Diabetes Ther (2024). https://doi.org/10.1007/s13300-024-01609-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13300-024-01609-3