Abstract
Introduction
Diabetes is a multifactorial disease with far-reaching consequences. Environmental factors, such as urban or rural residence, influence its prevalence and associated comorbidities. Haryana—a north Indian state—has undergone rapid urbanisation, and part of it is included in the National Capital Region (NCR). The primary aim of the study is to estimate the prevalence of diabetes in Haryana with urban–rural, NCR and non-NCR regional stratification and assess the factors affecting the likelihood of having diabetes among adults.
Methods
This sub-group analysis of the Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study (a nationally representative cross-sectional population-based survey) was done for Haryana using data from 3722 participants. The dependent variable was diabetes, while residence in NCR/non-NCR and urban–rural areas were prime independent variables. Weighted prevalence was estimated using state-specific sampling weights and standardized using National Family Health Survey-5 (NFHS-5) study weights. Associations were depicted using bivariate analysis, and factors describing the likelihood of living with diabetes were explored using a multivariable binary logistic regression analysis approach.
Results
Overall, the weighted prevalence of diabetes in Haryana was higher than the national average (12.4% vs. 11.4%). The prevalence was higher in urban (17.9%) than in rural areas (9.5%). The prevalence of diabetes in rural areas was higher in the NCR region, while that of prediabetes was higher in rural non-NCR region. Urban–rural participants’ anthropometric measurements and biochemical profiles depicted non-significant differences. Urban–rural status, age and physical activity levels were the most significant factors that affected the likelihood of living with diabetes.
Conclusions
The current analysis provides robust prevalence estimates highlighting the urban–rural disparities. Urban areas continue to have a high prevalence of diabetes and prediabetes; rural areas depict a much higher prevalence of prediabetes than diabetes. With the economic transition rapidly bridging the gap between urban and rural populations, health policymakers should plan efficient strategies to tackle the diabetes epidemic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Proximity to the national capital places Haryana at an elevated risk for diabetes and prediabetes. |
Urbanisation has reduced urban–rural disparities in diabetes, especially evident in the National Capital Region (NCR) and non-NCR areas of Haryana. |
The weighted prevalence of diabetes in Haryana exceeds the national average. |
Diabetes prevalence was notably higher in urban areas compared to rural areas, with targeted interventions recommended for the NCR and non-NCR regions in urban settings. |
A noteworthy prevalence of prediabetes in rural and urban locales within the non-NCR segment of Haryana presents a compelling focal point for further scientific investigation and intervention strategies. |
Introduction
Diabetes mellitus, a chronic metabolic disorder characterised by elevated blood glucose levels, remains a substantial public health concern worldwide. It is a leading cause of cardiovascular diseases, kidney failure, blindness and lower limb amputations [1]. In 2021, the global age-standardised total diabetes prevalence was 6.1% and is expected to reach 10% by 2050 if the current trend continues [2]. According to the International Diabetes Federation, approximately 537 million adults worldwide were living with diabetes in 2021, and this number is projected to be 784 million by 2045 [3]. The prevalence of diabetes and prediabetes remains slightly higher in India at 7.3% and 10.3%, respectively, with substantial variations at provincial levels [4]. The high burden in India equates to 8.5 million excess deaths, 42.7 million years of life lost (YLL) and 2.6 trillion US dollars in lost gross domestic product [5]. This alarming trend underscores the urgency of addressing diabetes and its associated complications, or else the rising burden can easily overwhelm the health system if not checked in time.
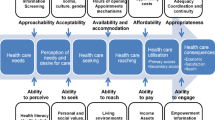
Diabetes is a complex and multifactorial disease with far-reaching consequences [6]. Of numerous factors contributing to the natural history of the disease, previous studies have shown a substantial difference in the prevalence of diabetes based on the resident’s regional and urban/rural status [7,8,9]. To address urban–rural disparities in diabetes prevalence, it is essential to delve into the contributing factors. Contrasting theories have been reported that explain the prevalence disparities in urban and rural areas. Existing literature corroborates a higher prevalence of diabetes in the urban populations compared to their rural counterparts and attributes this difference to a more sedentary lifestyle in the former, worsened by access to high-calorie, processed foods and increased stress in urban settings [10]. These factors exacerbate the risk of obesity and type 2 diabetes. Further, environmental factors such as air pollution and exposure to toxins have also been shown to impact diabetes risk. Urban areas often have higher pollution levels, which may further exacerbate diabetes risk [11].
At the same time, the prevalence of diabetes is also increasing in rural areas. Rural areas may have more physically demanding lifestyles and access to healthier, locally sourced foods. At the same time, some other studies explain higher prevalence in rural areas as a result of frequent barriers in accessing healthcare services due to geographical distances, limited healthcare facilities and workforce shortages. This can result in delayed diagnosis and inadequate management of diabetes. Further, it is known that lower socioeconomic status in rural areas, attributed to lower average incomes and reduced access to education and employment opportunities, contributes to higher diabetes prevalence [12]. Certain cultural norms and behaviours may contribute to the different rates of diabetes in urban and rural areas. However, rapid strides in communication and changes in lifestyle are contributing to decreasing differences in epidemiology in the two areas. A previous study depicted non-significant differences in the eating habits of urban and rural areas in Punjab—a northern state of India [13]. Therefore, studying urban–rural differences in the prevalence of diabetes and associated comorbidities in different sociocultural contexts seems essential.
Haryana is a northern state of India with common boundaries with Delhi’s National Capital Territory (NCT). The state has undergone a rapid economic transition over the last few years owing to the inclusion of most areas of Haryana as a part of the National Capital Region (NCR), a planning region centred upon the NCT in India which includes a mix of urban and rural areas. The development is substantial in urban and rural areas, leading to striking lifestyle changes on a similar scale. Therefore, it is intriguing to see the effect of economic development on the prevalence of diabetes and its risk factors in urban and rural areas, as well as NCR and non-NCR of Haryana. Within this context, the Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) research offers precise and thorough state- and national-level data on the prevalence of diabetes and other metabolic noncommunicable diseases in India, such as obesity, hypertension and dyslipidemia at the state and rural–urban levels through a cross-sectional, population-based survey of adults aged ≥ 20 years. Therefore, the primary aim of the study was to estimate the prevalence of diabetes in Haryana with urban–rural, NCR and non-NCR regional stratification and to assess the factors affecting the likelihood of having diabetes among adults as a sub-group analysis of the ICMR-INDIAB dataset.
Methods
Study Design
This is a sub-group analysis of the ICMR-INDIAB study, which was a cross-sectional study conducted in 31 states and Union-Territories/NCT of India in five phases by sampling 33,537 urban and 79,506 rural residents (overall n = 113,043) using a stratified multistage sampling design [14, 15].
Study Area
This study was conducted in Haryana during the fourth phase of the ICMR-INDIAB study between December 2018 and July 2019. Haryana is a gateway to North India, which occupies 1.37% of the total geographical area and account for less than 2% (25,351,462) of India’s population. It has 22 administrative districts, of which 14 are under the NCR. The primary occupation has been agriculture, the literacy rate is about 75% and is among the most prosperous states in India, having one of the highest per capita incomes in the country.
Sample Size and Sampling Design
The sample size for Haryana was calculated as 4000 (consisting of 2800 rural and 1200 urban inhabitants), after assuming an expected diabetes prevalence of 10% in urban and 4% in rural areas, allowing a relative precision of 20% of the estimated prevalence, an α error of 5% and a non-response rate of 20%. A three-level stratification based on each state’s geography, population size and socioeconomic status (SES) was used to obtain a representative sample. The primary sampling units were villages in rural areas and census enumeration blocks in urban areas. Using a systematic sampling method, 24 and 56 households were selected from urban and rural areas. The door-to-door assessment was done, and from each household, one individual was selected on the basis of the World Health Organization (WHO) Kish method to avoid selection bias with respect to sex and age. Finally, 3722 participants were included in the current analysis because of incomplete data.
Study Variables
Dependent Variable
The presence or absence of diabetes was the primary dependent variable without differentiating it into type 1 or 2 diabetes mellitus (DM). Fasting capillary blood glucose (CBG) was measured using a glucose meter (One Touch Ultra, Lifescan, Johnson & Johnson, Milpitas, CA, USA) after at least 8 h of overnight fasting. A participant was identified as living with DM if they had a physician diagnosis of diabetes or had high blood glucose levels i.e. capillary fasting blood glucose levels of at least 126 mg/dl, 2 h post-oral glucose load of ≥ 220 mg/dl, or both [16]. Prediabetes was defined as the presence of fasting blood glucose levels of ≥ 110 mg/dl or 2 h post-oral glucose load levels of ≥ 160 mg/dl. In individuals with self-reported diabetes, only fasting glucose was measured [16]. A venous sample was collected from the participants to assess the glycated hemoglobin (HbA1c) lipid profile (cholesterol, triglycerides, high-density lipoproteins) and serum creatinine.
Independent Variables
A pre-tested validated questionnaire collected information about the socioeconomic parameters and behavioural factors. The structured questionnaire was administered to 100 subjects for validation, and good reproducibility was observed after correlation analysis [14]. We included and categorised age (20–44, 45–59, and 60 and above), gender (male, female), residence (rural, urban), region of Haryana as per Government of Haryana notification (NCR and Non NCR regions) [17], religious beliefs (Hindu, others), marital status (currently in union, not in union), education status (nil, until primary, up to higher secondary, higher than secondary), occupation (manual labour, skilled workers: sale/clerical/managers/professionals, and unemployed), SES using 2011 revised Kuppuswamy’s scale for urban areas, and house type and the Standard of Living Index (SLI) per the National Family Health Survey-3 (NFHS-3) in rural areas (poor, middle class and upper class) [18], h/o hypertension as per the 7th report of the Joint National Committee [19], body mass index (BMI) using WHO Asia Pacific Guidelines to define overweight and obesity (i.e. normal or underweight, overweight/obese) [20, 21], physical activity levels (sedentary or light activity style, active or moderately active style, and vigorous or vigorous active style) as per Madras Diabetes Research Foundation Physical Activity Questionnaire (MPAQ) [22], current tobacco or alcohol user and consuming extra salt in the diet. Another section in the questionnaire collected information about participants’ knowledge of diabetes, organs affected and methods of prevention of diabetes. Anthropometric parameters (body weight, height, waist circumference and blood pressure) and biochemical parameters (cholesterol, triglyceride, high-density lipoproteins, serum creatinine) were measured using standard protocols [14, 15].
Statistical Analysis
Data were analysed using SPSS version 28.0. Sampling weights were derived on the basis of the design weight and individual response rate and normalised at the state level to obtain standard state weights as per the parent study. The details can be found elsewhere [23, 24]. Descriptive statistics were used to depict the estimates as weighted proportions or means ± standard deviations (SD). Furthermore, to achieve comparable estimates with the results from the primary study, re-weighted prevalence rates for urban–rural in NCR and non-NCR regions were achieved by multiplying the weighted prevalence of each sub-group from the ICMR-INDIAB dataset by the corresponding weight in the National Family Health Survey-5. Bivariate analysis was done using Student’s unpaired t tests, one-way analysis of variance (ANOVA) or chi-square tests. Adjusted odds ratios depicting the likelihood of having diabetes were derived along with their 95% confidence intervals (CI) using a multi-variable binary logistic regression analysis approach. A p value of < 0.05 was considered statistically significant for all statistical tests.
Ethical Approval
The study was ethically approved by the Institutional Ethics Committee of Madras Diabetes Research Foundation vide letter number MDRF/NCT/02-08/2018, dated 2 March 2018. Informed consent was obtained from all the participants before the interview was initiated, and their blood samples were drawn. Participants with abnormal biochemical profiles were ensured a standard of care by linking them to the nearest government health centre per pre-defined protocols. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Results
A total of 3722 participants (2345 in non-NCR and 1377 in NCR) were included in the final analysis. Table 1 shows the study participants’ sociodemographic characteristics and risk behaviours segregated by their NCR residential status. A large proportion of the participants were 20–44 years old (58.5%), married (82.3%), educated up to higher secondary (40%) and had low SES (44.0%). A significantly greater proportion of the participants in NCR districts resided in urban areas than those living in non-NCR districts. Other significant differences between NCR and non-NCR districts were religious beliefs, current tobacco use (higher in NCR regions), adding extra salt to the diet (higher in NCR) and awareness of diabetes (higher in non-NCR districts).
The overall weighted prevalence of diabetes, as depicted in Table 2, was 12.4% (95% CI 7.5–17.5). The prevalence was higher in urban areas (17.9%) than in rural areas (9.5%). The overall prevalence of prediabetes (18.2%, 95% CI 12.0–24.5) was greater than that of diabetes in both rural and urban areas. A similar trend was seen in both NCR and non-NCR regions, where prediabetes was more prevalent than diabetes in both urban and rural areas except the urban NCR region (Supplementary Table S1). Compared to rural areas, urban areas had a greater prevalence of diabetes in both NCR (9.7% vs. 19.5%) and non-NCR (8.9% vs. 14.0%) regions. Table 3 depicts the prevalence of diabetes in urban and rural areas per different sociodemographic characteristics. The prevalence of diabetes increased significantly with age in rural areas. But in urban areas, the prevalence was marginally higher in the 45–59 age group than ≥ 60 years. Diabetes was more prevalent among those who were illiterate and skilled workers (compared to manual labourers and unemployed) in rural areas. A significant proportion of people with high SES in both rural and urban areas were living with diabetes. High prevalence was seen among those who were overweight or obese, hypertensive and/or had a sedentary lifestyle, irrespective of the place of residence.
More people in urban than rural areas knew that diabetes can affect other organs. A significant proportion of people living with diabetes in rural areas thought that it was not preventable. More people in urban than rural areas knew about the preventive effect of diet and exercise on diabetes. The urban–rural differences in the weighted prevalence of diabetes per proximity to NCR are summarised in Supplementary Table S2. In the rural areas of the non-NCR region, diabetes was more prevalent among those who were unemployed and followed a religion other than Hindu. In the urban area of NCR region, diabetes was more prevalent among overweight or obese people.
Table 4 compares the anthropometric measurements and biochemical profile of all the participants, specifically those who were living with diabetes in urban and rural areas. The people living with diabetes in rural areas were significantly younger than those residing in urban areas. Compared to urban areas, the mean HbA1c levels were higher in rural areas (7.4 ± 2.3 vs. 7.9 ± 2.4, p = 0.66), but fasting glucose was lower (167.1 ± 69.7 vs. 152.7 ± 71.7, p = 0.54) among patients with diabetes. Overall, the anthropometric measurements and biochemical profile of urban–rural depicted non-significant differences and were comparable except for the serum creatinine levels (Table 4).
Table 5 depicts the multivariable binary logistic regression results and identifies the factors that affect the likelihood of living with diabetes. People living in rural areas are less likely to live with diabetes than in urban areas (aOR 0.6, 95% CI 0.5–0.7). Ageing was a significant predictor of diabetes. People aged 45–59 years and ≥ 60 years have a higher likelihood (aOR 2.3, 95% CI 1.8–2.9; and aOR 2.4, 95% CI 1.8–3.1) of living with diabetes than 20–44-year-olds. Compared to participants with a sedentary lifestyle, those doing vigorous physical activity had about 30% less likelihood of living with diabetes (aOR 0.7, 95% CI 0.3–0.9) times the risk of diabetes. Gender, education, occupation and SES were not significant predictors of diabetes. Other risk factors like alcohol, tobacco and high BMI did not significantly affect the odds of living with diabetes.
Discussion
With India being amongst the countries with the highest diabetes burden, it is crucial to concurrently focus on the clinical and epidemiological determinants to mitigate this silent public health problem. Our sub-national analysis presents some interesting findings. First, the weighted prevalence of diabetes in Haryana was higher than the national average (12.4% vs. 11.4%), even after segregating urban (17.9% vs. 16.4%) and rural (9.5% vs. 8.9%) data [15]. Second, the prevalence of diabetes was higher in urban areas, and even higher in the urban areas of NCR region. Third, the prevalence of diabetes in the rural areas was higher in the NCR region, but prediabetes was observed to be highest in the rural areas of the non-NCR region. Fourth, urban and rural participants’ anthropometric and biochemical profiles are comparable. Lastly, urban–rural status, higher age and lower physical activity levels were the most significant factors that affected the likelihood of living with diabetes.
The higher prevalence of diabetes in Haryana is a cause of concern. Meanwhile, the NFHS-5 estimates the prevalence of diabetes to be around 6.5% (95% CI 6.4–6.6) [25]. The differences in the estimates can be attributed to methodological differences. NFHS-5 checked ‘random sugar’ while both fasting and post-load glucose were measured in ICMR-INDIAB. Diabetes was more prevalent in urban areas than rural areas, irrespective of the proximity to the NCR region, similar to other studies [15, 26, 27]. Many studies have documented the rise in the prevalence of diabetes over time [26, 28]. A surveillance study conducted in Haryana found that 11.4% of men and 9.4% of women in urban areas and 3.9% of men and 1.6% of women in rural areas had diabetes in 2005. This has now escalated to the current estimates in a little over a decade, with a relative change of around 35% and 109.6% in urban areas and 158.9% and 656.5% in rural areas [28]. Furthermore, 9 in 50 participants had prediabetes (18.2%), which is also higher than the national average (15.3%) [15]. Prediabetes was on the rise in both rural and urban areas, with the highest prevalence reported in rural non-NCR regions (19.5%) followed by urban non-NCR regions (19.4%). However, no significant urban–rural differences in the prevalence of prediabetes were reported at the national level [15]. The mean fasting blood glucose has also increased in the population. In our study, the mean fasting capillary glucose (FCG) was 109.7 mg/dl and 102.1 mg/dl in urban and rural areas compared to 100.8 mg/dl in urban and 90 mg/dl in rural areas as per previous estimates [28]. These points towards greater impairment of fasting glucose and a higher risk of prediabetes.
We observed significant socioeconomic disparities in the prevalence of diabetes. The risk of diabetes increases with age [4, 29, 30]. People ≥ 60 years had 2.4 times the risk of diabetes than the 20–44 years age group. Many factors explain the increasing prevalence with age [31]. Contrary to the findings from the national study, women reported a higher prevalence than men in the current study [23]. However, the effect of sex on the prevalence was non-significant on logistic regression. Most previous studies report a higher prevalence of diabetes in men [4, 29, 30]. The rising prevalence in women reflects the lesser protective effect of female physiology, as stated in the past [28]. Changing lifestyles, dietary patterns and obesity in women may also contribute to this trend. The sex differences in the prevalence of diabetes are reversed according to the stage of reproductive life, and there are more women with diabetes after menopause. Also, women show more impaired glucose tolerance after a meal. Therefore, considering only fasting blood glucose as a screening and excluding post-prandial blood glucose values in calculating the prevalence of diabetes in women can lead to underestimating a much more evident phenomenon [11]. Diabetes was more prevalent among the illiterate participants. In other studies, educational status has also been significantly linked to diabetes [26, 30]. The high prevalence of diabetes in urban areas may be attributed to awareness about diabetes, increased screening and better health facilities. Living in an urban area was an independent predictor of diabetes, similar to other studies [4, 26, 29]. Compared to the poor, people with high SES are at a greater risk of diabetes, but not in our study [4].
Obesity and a sedentary lifestyle are a result of economic transition and urbanisation. The prevalence of obesity was higher among people living with diabetes in urban areas (20.7%) than in rural areas (12.8%). Both abdominal and generalised obesity increases the risk of diabetes, especially among Indians [4, 26, 29, 32]. However, obesity was not a significant predictor in our study. Vigorous physical activity was protective against diabetes. A similar finding was observed in another study where people following a sedentary lifestyle had 2.3 times (1.95–2.71) the rates of self-reported diabetes than those doing vigorous activity [26]. Lack of physical activity may lead to obesity, increasing the risk of diabetes. Smoking and alcohol were not related to diabetes in our study. A considerable proportion of people are at risk of developing diabetes in future. The high prevalence of prediabetes coupled with poor access to health services will contribute to a rise in morbidity and mortality due to diabetes in rural areas. This calls for greater investment in screening and early diagnosis of the syndrome.
This study has particular strengths and limitations. It is amongst the largest studies with a representative sample from Haryana, making the results policy-relevant and generalisable. Data were collected by trained investigators using standard techniques, which made the results reliable. The methodology restricted us from differentiating between type 1 and type 2 diabetes. The prevalence was based on a single reading and capillary blood glucose estimation and not venous blood samples as a result of logistic constraints. However, the current analysis was limited by the number of variables in the original survey. Apart from hypertension and obesity, no other comorbidities were considered in this analysis. Also, we did not comprehensively ascertain the role of dietary patterns on diabetes prevalence in urban and rural areas, as it is a relevant component necessitating separate evaluation. Furthermore, our knowledge continues to expand about different variables that can affect diabetes epidemiology, PM2.5 and gut microflora being some of them. The cross-sectional nature of the study prevents us from making causal inferences.
Our study results have specific policy implications and recommendations emerging from it. By comprehensively examining these disparities and the underlying factors, we can pave the way for more effective prevention, management and policy initiatives. Our study depicts specific interesting findings that can inform the development of interventions to address the unique needs of urban and rural populations. Stakeholders can use this research to draft policies addressing the root causes of urban–rural disparities in diabetes and comorbidities, such as improving healthcare infrastructure in rural areas and implementing strategies to promote healthier lifestyles. Understanding the cultural and behavioural factors influencing diabetes risk can guide the development of targeted health education programs that resonate with urban and rural communities. The increased prevalence in rural areas is concerning and demands more efforts through better public health preparedness, regular screening, uninterrupted supply of medicines, and logistics support to screen for emerging micro- and macrovascular complications.
Conclusion
We depict a higher prevalence of diabetes in Haryana than the national average. While urban areas continue to have a higher prevalence of diabetes and prediabetes, rural areas may catch up because of an increase in the prevalence of prediabetes, which must be addressed promptly. As economic transition rapidly bridges the gap between urban and rural populations, the health system should also gear up to identify risk factors and tackle them in all regions of the state. Timely measures can efficiently contribute to improved public health outcomes and a reduction in the burden of this chronic disease on individuals and healthcare systems alike.
Data Availability
Raw data are available on reasonable request to the corresponding author.
References
Hippisley-Cox J, Coupland C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. BMJ. 2016. https://doi.org/10.1136/bmj.i1450.
Ong KL, Stafford LK, McLaughlin SA, et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;402(10397):203–34.
International Diabetes Federation. Diabetes atlas 10th edition. 2021. https://diabetesatlas.org/data/en/country/93/in.html. Accessed 16 June 2023.
Anjana RM, Deepa M, Pradeepa R, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5(8):585–96.
Banker KK, Liew D, Ademi Z, et al. The impact of diabetes on productivity in India. Diabetes Care. 2021;44(12):2714–22.
Ranasinghe P, Jayawardena R, Gamage N, Sivanandam N, Misra A. Prevalence and trends of the diabetes epidemic in urban and rural India: a pooled systematic review and meta-analysis of 1.7 million adults. Ann Epidemiol. 2021;58:128–48.
Biswas T, Islam A, Rawal LB, Islam SMS. Increasing prevalence of diabetes in Bangladesh: a scoping review. Public Health. 2016;1(138):4–11.
O’connor A, Wellenius G. Ruraleurban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012. https://doi.org/10.1016/j.puhe.2012.05.029.
Gebremedhin G, Enqueselassie F, Deyessa N, Yifter H. Urban-rural differences in the trends of type 1 and type 2 diabetes among adults who received medical treatment from public hospitals in resource-poor community Tigray, Ethiopia. Diabetes Metab Syndr Obes. 2020;13:859–68.
Velmurugan G, Mohanraj S, Dhivakar M, et al. Differential risk factor profile of diabetes and atherosclerosis in rural, sub-urban and urban regions of South India: the KMCH-Non-communicable disease studies. Diabet Med. 2021. https://doi.org/10.1111/dme.14466.
Mandal S, Jaganathan S, Kondal D, et al. PM 2.5 exposure, glycemic markers and incidence of type 2 diabetes in two large Indian cities. BMJ Open Diabetes Res Care. 2023;11(5):e003333. https://doi.org/10.1136/bmjdrc-2023-003333.
Barman P, Das M, Verma M. Epidemiology of type 2 diabetes mellitus and treatment utilization patterns among the elderly from the first wave of Longitudinal Aging study in India (2017–18) using a Heckman selection model. BMC Public Health. 2023. https://doi.org/10.1186/s12889-023-15661-4.
Verma M, Aggarwal R, Nath B, Kakkar R. Exploring the influence of food labels and advertisements on eating habits of children: a cross-sectional study from Punjab, India. BMC Public Health. 2023. https://doi.org/10.1186/s12889-023-15058-3.
Anjana RM, Pradeepa R, Deepa M, et al. The Indian Council of Medical Research—India Diabetes (ICMR-INDIAB) study: methodological details. J Diabetes Sci Technol. 2011;5(4):906–14.
Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474–89.
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Report of a WHO/IDF Consultation. 2006. https://www.who.int/publications/i/item/definition-and-diagnosis-of-diabetes-mellitus-and-intermediate-hyperglycaemia. Accessed 25 Dec 2023.
National Capital Region Planning Board. 2024. https://ncrpb.nic.in/. updated on 3 May, 2024.
Demographic Health Survey. Wealth index construction. The DHS program. 2016. https://dhsprogram.com/topics/wealth-index/Wealth-Index-Construction.cfm. Accessed 26 Feb 2022.
Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–52. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
Misra A. Ethnic-specific criteria for classification of body mass index: a perspective for Asian Indians and American Diabetes Association position statement. Diabetes Technol Ther. 2015;17(9):667–71.
Organization WH. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000.
Anjana RM, Sudha V, Lakshmipriya N, et al. Reliability and validity of a new physical activity questionnaire for India. Int J Behav Nutr Phys Act. 2015;12(1):1–12. https://doi.org/10.1186/s12966-015-0196-2.
Anjana RM, Unnikrishnan R, Deepa M, et al. Metabolic non-communicable disease health report of India: the ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17). Lancet Diabetes Endocrinol. 2023;11(7):474–89.
Anjana RM, Pradeepa R, Deepa M, et al. The Indian Council of Medical Research—India Diabetes (ICMR-INDIAB) study: methodological details. J Diabetes Sci Technol. 2011;5(4):906–14. https://doi.org/10.1177/193229681100500413.
Varghese JS, Anjana RM, Geldsetzer P, et al. Diabetes diagnosis, treatment, and control in India: results from a national survey of 1.65 million adults aged 18 years and older, 2019–2021. medRxiv. 2023
Mohan V, Mathur P, Deepa R, et al. Urban rural differences in prevalence of self-reported diabetes in India-The WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80(1):159–68.
Deepa M, Anjana RM, Manjula D, Narayan KMV, Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai urban population study. J Diabetes Sci Technol. 2011;5(4):918.
Nongkynrih B, Acharya A, Ramakrishnan L, Ritvik AK, Shah B. Profile of biochemical risk factors for non communicable diseases in urban, rural and periurban Haryana, India. J Assoc Physicians India. 2008;56:165–70.
Anjana RM, Pradeepa R, Deepa M, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54(12):3022–7.
Das U, Kar N. Prevalence and risk factor of diabetes among the elderly people in West Bengal: evidence-based LASI 1st wave. BMC Endocr Disord. 2023. https://doi.org/10.1186/s12902-023-01421-3.
Pradeepa R, Mohan V. Epidemiology of type 2 diabetes in India. Indian J Ophthalmol. 2021;69(11):2932.
Misra R, Madhavan SS, Dhumal T, Sambamoorthi U. Prevalence and factors associated with diagnosed diabetes mellitus among Asian Indian adults in the United States. PLOS Glob Public Health. 2023;3(2):e0001551.
Funding
This is a subgroup analysis of a larger study that was funded by Indian Council of Medical Research and Department of Health Research, Ministry of Health and Family Welfare, Government of India. No funding or sponsorship was received for publication of this article.
Author information
Authors and Affiliations
Contributions
Sanjay Kalra, Viswanathan Mohan, Ranjit Mohan Anjana, Madhur Verma contributed to study concept and design of the study; Madhur Verma, Nikita Sharma, Omna Singh, Rajendra Pradeepa, Mohan Deepa, Ulagamadesan Venkatesan and Nirmal Elangovan were involved in the data analysis, interpretation of the results, and writing the first draft of the study; Sanjay Kalra, Ranjit Mohan Anjana, Madhur Verma, Sameer Aggarwal, Rakesh Kakkar, and Viswanathan Mohan contributed to the development of manuscript and revising it extensively. All the authors have read and approved the final version of the manuscript. Sanjay Kalra is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of Interest
Sanjay Kalra is an Editorial Board member of Diabetes Therapy. Sanjay Kalra was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. The other authors (Ranjit Mohan Anjana, Madhur Verma, Rajendra Pradeepa, Nikita Sharma, Mohan Deepa, Omna Singh, Ulagamadesan Venkatesan, Nirmal Elangovan, Sameer Aggarwal, Rakesh Kakkar and Viswanathan Mohan) have nothing to disclose.
Ethical Approval
The study was ethically approved by the Institutional Ethics Committee of Madras Diabetes Research Foundation vide letter number MDRF/NCT/02–08/2018, dated 2 March 2018. Informed consent was obtained from all the participants before the interview was initiated, and their blood samples were drawn. Participants with abnormal biochemical profiles were ensured a standard of care by linking them to the nearest government health centre per pre-defined protocols. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Kalra, S., Anjana, R.M., Verma, M. et al. Urban–Rural Differences in the Prevalence of Diabetes Among Adults in Haryana, India: The ICMR-INDIAB Study (ICMR-INDIAB-18). Diabetes Ther 15, 1597–1613 (2024). https://doi.org/10.1007/s13300-024-01602-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01602-w