Abstract
Introduction
In patients with type 2 diabetes (T2D), treatment with sodium–glucose cotransporter-2 (SGLT2) inhibitors has been shown to reduce hospital admission rates for heart failure (HF). However, the multiple mechanisms hypothesized and investigated to explain the cardioprotection of SGLT2 inhibitors are not fully understood.
Objectives
The effect of luseogliflozin on myocardial flow reserve (MFR) in patients with T2D (LUCENT-J) study aims to examine the effects of SGLT2 inhibitors on myocardial perfusion.
Methods
The LUCENT-J study is a prospective, single-center, randomized, two-arm, parallel-group, open-label (i.e., the radiology readers are blinded), active-controlled study. A cohort of 40 patients with T2D with no or stable (with no history of myocardial infarction and with or without previous percutaneous coronary intervention) coronary artery disease will be included. Patients will be randomized in a 1:1 ratio to luseogliflozin or control and treated for 24 weeks. The primary outcome is the change in MFR, as measured by 13N-ammonia positron emission tomography/computed tomography, from baseline to 24 weeks after treatment initiation.
Planned Outcomes
The LUCENT-J study will elucidate the mechanisms of cardioprotection by SGLT2 inhibitors in patients with T2D.
Trial Registration
Japan Registry of Clinical Trials (JRCTs051220016).
Similar content being viewed by others
Heart failure (HF) is highly prevalent in people with type 2 diabetes (T2D) and is an indicator of poor prognosis. |
Sodium–glucose cotransporter-2 (SGLT2) inhibitors reduce hospitalization rates for HF. |
The mechanisms underlying the cardioprotective effects of SGLT2 inhibitors are insufficiently understood. |
This study aimed to elucidate the effects of SGLT2 on cardiac microcirculation and myocardial flow reserve using 13N-ammonia positron emission tomography and to help understand how SGLT2 inhibitors prevent HF. |
Introduction
Sodium–glucose cotransporter-2 (SGLT2) inhibitors act on the proximal renal tubules to reduce glucose, thereby lowering blood glucose. These drugs were initially developed as antihyperglycemic agents. However, the EMPA-REG trial demonstrated for the first time that a glucose-lowering drug, the SGLT2 inhibitor empagliflozin, both rapidly and significantly decreased the risk of hospitalization for HF and cardiovascular death in patients with T2D [1]. Subsequently, the DECLARE-TIMI58 and CANVAS trials demonstrated the cardiovascular disease benefits of the SGLT2 inhibitor in patients with T2D [2, 3]. The multiple mechanisms hypothesized and investigated to explain the beneficial cardiovascular effects of the SGLT2 inhibitor are the subject of continuous investigation. Furthermore, the DAPA-HF and EMPEROR-Reduced trials showed that in patients with and without T2D, dapagliflozin and empagliflozin improved cardiovascular outcomes [4, 5]. The pleiotropic effects of SGLT2 inhibitors have been shown to reduce the incidence and progression of HF and improve the prognosis of HF.
The mechanisms that appear to play a major role in these functions are (1) natriuresis and osmotic diuresis, (2) inhibition of the sympathetic nervous system, (3) improvement in myocardial energy metabolism, and (4) renal protection. Increased natriuresis and osmotic diuresis have been shown to reduce plasma volume, and improved endothelial function, reduced arterial stiffness, and reduced blood pressure may reduce both preload and afterload of the heart [6, 7].
The reduction in blood pressure in the absence of increasing heart rate caused by SGLT2 inhibitors may be indirectly associated with a reduction in sympathetic nervous system activity [8]. SGLT2 inhibitor treatment prevents the development of salt-induced blood pressure elevation and abnormality of the blood pressure circadian rhythm.
The loss of glucose through urine results in increased plasma levels of ketones. The increased use of ketones for energy production improves the energy supply to the “starving” failing heart [9,10,11]. There are several important interactions between heart disease and kidney disease. These interactions have been referred to as “cardiorenal syndrome” and exacerbation of HF. Several trials have reported renal protection after SGLT2 inhibitor treatment. Treatment with SGLT2 inhibitors was observed to attenuate renal hyperfiltration by affecting tubular–glomerular feedback mechanisms [12].
Diabetes is a risk factor for atherosclerosis, and HF develops from angina pectoris or myocardial infarction due to reduced left ventricular systolic function (HF with reduced ejection fraction). Conversely, patients with diabetes have diastolic dysfunction despite the absence of coronary artery lesions (HF with preserved ejection fraction; HFpEF). Myocardial microcirculatory dysfunction has been implicated in HFpEF [13, 14].
Cardiac microcirculation can be investigated noninvasively by 13N-ammonia positron emission tomography (PET). 13N-ammonia PET examines myocardial flow reserve (MFR), which is the ratio between myocardial blood flow at rest and at stress-induced hyperemic conditions pharmacologically. In addition to organic coronary artery lesions, microcirculatory dysfunction can also be detected. MFR has been reported to be reduced by dipyridamole loading of 13N-ammonia PET in patients with T2D [15].
The use of coronary flow reserve as a functional indicator of coronary artery stenosis by cardiac catheterization is useful and has been reported, for example, in the FAME study as an indicator for assessing vascular lesions at the conduit vessel site, whereas MFR represents the effect of microcirculation as well as conduit vessels.
Therefore, in addition to the ischemia diagnosis, the MFR of the whole myocardium has been reported to be very useful for predicting prognosis regardless of the underlying HF [16,17,18,19]. 13N-ammonia PET studies can be used to assess not only ischemia but also microcirculatory effects.
This may be important for elucidating the mechanisms underlying the effects of SGLT2 inhibitors in HF. In this study, we investigate the effect of luseogliflozin administration on MFR as a cardioprotective mechanism by SGLT2 inhibitors.
Adrenomedullin is a bioactive peptide with potent vasodilatory properties discovered in brown cell tissue, which is elevated in cardiovascular disease and has physiological effects, including cardiovascular protection [20, 21].
In this study, we will measure 13N-ammonia PET and plasma adrenomedullin levels before and after luseogliflozin administration to analyze and investigate the improvement in myocardial microcirculatory dysfunction and to clarify some of the mechanisms of cardioprotection in diabetes. We hypothesize that improving microcirculatory dysfunction is a mechanism of cardioprotection with SGLT2 inhibitors.
Methods
Study Design
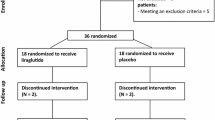
This is a prospective, single-center, randomized, two-arm, parallel-group, open-label (i.e., the radiology readers are blinded), active-controlled study conducted in patients attending the Division of Diabetes and Lipid Metabolism at the National Cerebral and Cardiovascular Center in Japan. The study aimed to evaluate the effect of luseogliflozin on MFR using 13N-ammonia PET in patients with T2D after 24 weeks of treatment (Fig. 1). Qualifying patients will be randomly assigned to receive luseogliflozin or conventional therapy. In the luseogliflozin group, luseogliflozin will be administered orally in addition to the participant’s ongoing diabetic treatment. In the control group, participants will only receive non-SGLT2 inhibitors as ongoing treatment (see the “Treatments” section below).
Study design. A total of 40 patients with type 2 diabetes mellitus (20 patients/group) will participate in this study. After patient eligibility is confirmed by a central managing modality, the enrolled patients will be randomly assigned to the luseogliflozin or control group. In the luseogliflozin group, 2.5 mg luseogliflozin will be administered orally once per day. In the control group, non–sodium–glucose cotransporter-2 (SGLT2) inhibitor treatment will continue. If necessary, the luseogliflozin dose will be increased to up to 5 mg per day
Ethics Compliance
This study will be conducted in compliance with both the articles of the Declaration of Helsinki (revised in October 2013) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labor and Welfare in Japan. The registration period is from April 28, 2022, to August 31, 2023, and the research period is from April 28, 2022, to May 31, 2024. In accordance with the law for clinical research in Japan, the Nara Medical University Certified Review Board approved the study protocol (approval No. nara0050). Written informed consent has been obtained from all participants.
Planned Outcomes
Primary Endpoint
The primary endpoint of the study is the difference between the groups in the amount of MFR change 24 weeks after initiation of administration.
Secondary Endpoints
-
1.
The amount of change in the following items within each group and the difference between the groups at the time of each measurement (4, 12, and 24 weeks) from the start of the study: body weight, body mass index (BMI), systolic blood pressure, diastolic blood pressure, hemoglobin, hematocrit, total protein, albumin, total bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), glutamic pyruvic transaminase (ɤ-GTP), serum creatinine, estimated glomerular filtration rate (eGFR), lactate dehydrogenase (LDH), calcium (Ca), sodium (Na), potassium (K), chlorine (Cl), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), uric acid, C-reactive protein (CRP), fasting blood sugar (FBS), immunoreactive insulin (IRI), HbA1c, acetoacetic acid, β-hydroxybutyric acid, and urinary albumin creatinine ratio. The amount of change in the following items within each group and the difference between the groups up to 24 weeks from the initiation of administration: B-type natriuretic peptide (BNP), adrenomedullin, and left ventricular ejection fraction (LVEF) assessed by 13N-ammonia PET.
-
2.
Difference in the amount of change in MFR between the groups with high and low MFR values before administration.
Study Population
The study will include 40 patients with T2D attending the Division of Diabetes and Lipid Metabolism at the National Cerebral and Cardiovascular Center, with HbA1c < 8.5%, under glycemic control by diet, exercise, and with/without pharmacotherapy (except SGLT2 inhibitors) as appropriate. Patients should also be able to provide written informed consent and have stable (no history of myocardial infarction, with or without previous percutaneous coronary intervention) or no coronary artery disease. Table 1 details the inclusion and exclusion criteria.
Randomization
Eligible and consenting participants will be randomized in a 1:1 ratio to luseogliflozin or control using the following assignment factors: sex, age (< 70 or ≥ 70 years), HbA1c (< 8.0 or ≥ 8.0%), MFR (< 1.9 or ≥ 1.9), and whether or not a glucagon-like peptide-1 (GLP-1) receptor agonist will be administered using the web-based minimization dynamic allocation method.
Treatments
In the luseogliflozin group, 2.5 mg of luseogliflozin (Lusefi® tablets 2.5 mg) will be administered orally in addition to the participant’s ongoing diabetic treatment. If the effect is insufficient, the dose can be increased to 5 mg (Lusefi® tablets 5 mg) per day. In the control group, treatment with non-SGLT2 inhibitors is continued. Each patient’s blood glucose level will be controlled according to the blood glucose control goals established by the Japan Diabetes Society at the discretion of the diabetes specialist.
As far as possible, diabetic and antihypertensive drugs will be continued without modification from the date of informed consent until the date of study initiation. During the treatment period, nitroglycerin, vasodilators, and GLP-1 receptor agonists will not change. However, such events will be recorded if they occur.
MFR 13N Ammonia PET CT
Rest and pharmacological stress PET scanning is conducted using a digital PET/CT system (Discovery MI, GE Healthcare). The procedure is started with a low-dose transmission CT scan for attenuation correction. A 20-frame (12 × 10 s, 6 × 30 s, 2 × 60 s, and 1 × 180 s) three-dimensional dynamic emission scan is initiated simultaneously with the intravenous administration of 370 MBq of 13N ammonia over 30 s using an automatic injector. After approximately five half-lives of 13N ammonia had elapsed, pharmacological stress imaging is conducted identically, with a preceding infusion of adenosine (140 μg/kg/min) administered 3 min before 370 MBq of 13N ammonia is intravenously injected automatically over 30 s. The adenosine infusion is continued for 6 min.
The dynamic image dataset is processed using two commercially available pharmacokinetic software packages: syngo myocardial blood flow (MBF) version VB15 (Siemens Medical Solutions) and Corridor 4DM (INVIA Medical Imaging Solutions). MBF is estimated using the time–activity curve (TAC) of the left ventricle input and myocardial uptake in compartment models. MFR is determined as the ratio of adenosine stress hyperemic MBF to resting MBF, with MFR < 2.0 considered abnormal. The MBF and MFR results are expressed in each major coronary artery territory and segment according to the AHA’s 17-segment model.
Measurements
Table 2 details the data collection schedule.
Sample Size Calculation
We based the sample size calculation on the published results of a previous trial on the effect of improvement in blood glucose with diabetic drugs on MFR in patients with T2D [22]. Taking into consideration an expected delta of MFR of 0.13 mL/min/g and a standard deviation of 0.16, 16 patients per treatment group are considered a sufficient number to reject the null hypothesis that the population means of the two groups are equal with a power of 85% and an alpha of 0.05. Considering a 20% study dropout rate, the target number of patients was set to 20 per group for 40 patients. Given the small sample size, a high dropout rate was set to account for the large number of dropouts, which would have a considerable impact on the analysis results.
Data Analysis
Analysis Populations
Two analysis groups were defined for the evaluation of effectiveness: a full analysis set (FAS) and a per-protocol set (PPS), with the FAS used for the primary analysis. The safety analysis set will include patients for whom safety assessment data were collected after the start of treatment in the study.
-
FAS is defined as the population of participants who provided informed consent and had a measured value for the factors of the primary endpoint at baseline and at least one time point during the treatment period.
-
PPS is defined as the population of participants for whom a post-study review did not identify any considerable deviation from the protocol.
Statistical Methods
The primary endpoint will be the difference in the change in MFR from baseline to 24 weeks. The difference in change in MFR from baseline to 24 weeks between the two groups will be tested by analysis of covariance, and summary statistics will be calculated. The covariates will be gender, age, MFR, HbA1c, and GLP-1 receptor agonist. We set the significance level at 0.05 two-sided. We will also perform the same test using BMI added to the covariates as a sensitivity analysis. Summary statistics will also be calculated for uncorrected cases as reference values. As secondary endpoints, we will evaluate the amount of change within each group and the difference in change between groups (those not measured at 4 and 12 weeks only after 24 weeks), and we will calculate the summary statistics quantities for the following items: body weight, BMI, systolic blood pressure, diastolic blood pressure, hemoglobin, hematocrit, HbA1c, FBS, IRI, blood acetoacetic acid, β-hydroxybutyric acid, total protein, albumin, total bilirubin, serum creatinine, serum uric acid, eGFR, AST, ALT, ɤ-GTP, LDH, LDL-C, TG, HDL-C, Na, K, Cl, Ca, CRP, BNP, adrenomedullin, urinary albumin creatinine ratio, and LVEF. In each group, the change from baseline to 4, 12, and 24 weeks will be tested for each test item. The test will be either paired t test or Wilcoxon signed-rank test. The significance level will be two-sided 0.05. We will compare the change from baseline between the luseogliflozin group and the control groups. Tests will be Student’s t test or Wilcoxon rank-sum test. The significance level will be set at 0.05. Subgroup analysis will compare the difference in change in MFR between the luseogliflozin group and the control group in each of the above- and below-median pretreatment MFR data populations. We will test the difference in the change in MFR from baseline to 24 weeks between the two groups by analysis of covariance, and summary statistics will be calculated. The covariates will be gender, age, HbA1c, and use of GLP-1 receptor agonist. The significance level will be set at two-sided 0.05. We will also perform the same test using BMI added to the covariates as a sensitivity analysis.
Strengths and Limitations
This is a single-center study with a relatively small number of participants. The main strength of this study is the highly refined gold standard method for assessing MFR by 13N-ammonia PET. Furthermore, the highly selected study population includes patients with T2D with a narrow HbA1c range (HbA1c ≤ 8.5%) who do not require revascularization or who are clinically stable after percutaneous coronary intervention. Therefore, the number of eligible patients is limited by the inclusion criteria, which are time-consuming and costly. However, with the right patients, accurate assessments could be made, and study results could lead to further cardioprotective studies in more patients with diabetes.
Conclusions
This study will elucidate the effects of SGLT2 on cardiac microcirculation. The results of this study will provide insights into the mechanism by which SGLT2 inhibitors prevent HF.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099.
McMurray JJV, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Packer M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24.
Yasui A, et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018;9:863–71.
Nassif ME, et al. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE—HF Trial. Circulation. 2021;143:1673–86.
Cherney DZ, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28.
Verma S, et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. JACC Basic Transl Sci. 2018;3:575–87.
Ferrannini E, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–5.
Koutnik AP, D’Agostino DP, Egan B. Anticatabolic effects of ketone bodies in skeletal muscle. Trends Endocrinol Metab. 2019;30:227–9.
Cice G, Calo L, Monzo L, Caló L. Sodium-glucose co-transporter 2 inhibitors for the treatment of cardio-renal syndrome. Eur Heart J. 2022;24(Suppl I):I68–71.
Levelt E, et al. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J. 2016;37:3461–9.
Shah SJ, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;1(39):3439–50.
Kjaer A, Meyer C, Nielsen FS, Parving HH, Hesse B. Dipyridamole, cold pressor test, and demonstration of endothelial dysfunction: a PET study of myocardial perfusion in diabetes. J Nucl Med. 2003;44:19–23.
van de Hoef TP, et al. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–11.
Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. 2018;27(72):2625–41.
Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging. 2009;2:1009–23.
Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–40.
Voors AA, et al. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail. 2019;21:163–71.
Ter Maaten JM, et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur J Heart Fail. 2019;21:732–43.
Schindler TH, et al. Improvement in coronary vascular dysfunction produced with euglycaemic control in patients with type 2 diabetes. Heart. 2007;93:345–9.
Acknowledgements
The authors thank the participants of the study and all of the clinical staff at the Division of Diabetes and Dyslipidemia, National Cerebral and Cardiovascular Center for their assistance, especially Maki Maruki, Emi Kanai, Kanami Sai, Noriko Umeda, Keiko Ishikawa, Satsuki Okui, Naoko Yamashita, and Tomona Yamamoto. In addition, the authors would like to thank Shido Co., Ltd. for data management and statistical analyses.
Medical Writing/Editorial Assistance
Enago was used for manuscript review and editing support and was funded by the Department of Advanced Cardiovascular Prevention and Epidemiology, Tohoku University Graduate School of Medicine and Tohoku University Hospital.
Funding
Taisho Pharmaceutical Co., Ltd. funded this study and the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
All authors are involved in the planning and execution of the LUCENT-J study. Tamiko Tamanaha is mainly responsible for drafting the manuscript with the help of Hisashi Makino, Cheol Son, and Kiminori Hosoda, who especially contributed to study planning. Yoshihiro Miyamoto and Satoshi Yasuda supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
Tamiko Tamanaha, Hisashi Makino, Cheol Son, Ryo Koezuka, Mayu Tochia, Yoko Ohata, Tatsuya Takekawa, Michio Noguchi, Tsutomu Tomita, Kyoko Honda-Kohmo, Miki Matsuo, Emi Tateishi, Tetsuya Fukuda, Yoshihiro Miyamoto, and Kiminori Hosoda have nothing to disclose.
Ethical approval
The study protocol was approved by the Nara Medical University Certified Review Board (approval No. nara0050) and will be conducted in compliance with both the articles of the Declaration of Helsinki (revised in October 2013) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Health, Labour and Welfare in Japan. Written informed consent has been obtained from all participants.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tamanaha, T., Makino, H., Son, C. et al. Effect of Luseogliflozin on Myocardial Flow Reserve in Patients with Type 2 Diabetes Mellitus (LUCENT-J Study). Diabetes Ther 15, 1245–1254 (2024). https://doi.org/10.1007/s13300-024-01571-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01571-0