Abstract
Introduction
Diabetes is associated with significant economic burden. Moreover, cardiovascular disease (CVD), including heart failure, and chronic kidney disease (CKD) are common comorbidities, leading to premature mortality. We conducted a systematic review to assess the humanistic and economic burden of cardio-renal-metabolic (CRM) conditions in individuals ≥ 18 years with CVD, CKD, and type 2 diabetes mellitus.
Methods
We searched Embase® and Medline® databases from 2011 to January 10, 2022 for English publications reporting humanistic and economic burden outcomes from observational studies, real-world evidence, and economic model studies. Intervention and validation studies were excluded. Study quality was assessed using the Newcastle–Ottawa Scale. Abstracts/posters were identified from four conferences (2020–2022).
Results
Of 1804 studies identified, 22 (including four conference publications) were selected involving 351,296,930 participants (one modeled the US population); eight reported healthcare resource utilization (HCRU), seven only cost data, six HCRU and cost data, one reported quality-of-life data (11/18 and 7/18 had estimated low and medium risk of bias, respectively). Participants were predominantly ≥ 65 years and identified as having White ethnicity. Higher costs and HCRU were observed in patients with all three conditions compared to those with two or none. Urban/metropolitan and insured patients had higher healthcare expenditure and service utilization compared to uninsured and racial/ethnic minority populations. Comorbidities were associated with increased hospitalizations, higher costs, and more emergency department visits. In general, patients identified as having Black ethnicity had low odds of using healthcare services, possibly due to disparities in healthcare access and distrust in the system. Limitations included no adjustment for inflation and a predominance of retrospective studies.
Conclusions
This review showed a greater economic burden for patients with CRM conditions, with a clear trend between increasing numbers of comorbidities and increasing healthcare costs/resource use. Comparisons between countries are complicated and the scarcity of evidence from minority racial and ethnic groups and lack of data from non-US geographies warrant further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This systematic review assessed the humanistic and economic burden of cardio-renal-metabolic (CRM) conditions in individuals ≥ 18 years with cardiovascular disease (CVD), chronic kidney disease (CKD), and type 2 diabetes mellitus. |
Limited research data are available on the economic burden of all three CRM conditions together, with a particular paucity of information on the humanistic burden in patients with CRM. |
In patients with diabetes, higher costs and utilization of healthcare resources were observed in patients with increasing number of comorbidities, and were associated with more hospitalizations, longer lengths of stay, and more visits to the emergency department. |
Uninsured patients and those belonging to US racial and ethnic minority populations have lower odds of utilizing healthcare services, possibly due to a lack of disease awareness, differences in access to healthcare, and distrust in the healthcare system. |
Targeted interventions, such as early and more aggressive lifestyle changes and evidence-based pharmacological treatments, are warranted for patients with multiple CRM comorbidities to improve outcomes, quality of care, and cost efficiencies across healthcare systems. |
Introduction
The International Diabetes Federation estimated that 10% of adults (20–79 years old) worldwide had diabetes in 2021 (i.e., 537 million people); this number is predicted to rise to 783 million by 2045 [1]. In the United States (US), an estimated 32 million adults had diabetes in 2021, with a projected increase to 36 million by 2045 [1]. Type 2 diabetes mellitus (T2D) accounts for 90–95% of diagnosed cases, with major complications/co-morbidities being hypertension and dyslipidemia, atherosclerotic cardiovascular disease (ASCVD) [2], heart failure (HF) [3,4,5], and chronic kidney disease (CKD) [6,7,8].
Insights from epidemiological, clinical, and basic research have highlighted the closely intertwined influence of metabolic disorders, cardiovascular diseases (CVDs; including HF), and kidney dysfunction, collectively referred to as cardio-renal-metabolic (CRM) conditions [9]. These conditions reflect multidirectional pathophysiologic interactions that increase CRM risk, while representing individual diseases that are complications of one another. In fact, 94% of people with T2D had ≥ 1 other CRM conditions and 51% had ≥ 3 other CRM conditions (commonly, combinations of hypertension, hyperlipidemia, ASCVD, and CKD) [10]. The 10-year incidence of cardiovascular death was 2–3 times higher in patients with either T2D (6.7%) or CKD (9.9%) than in those without either condition (3.4%), and fivefold higher in those with both conditions (19.6%) [11]. Similarly, an observational study of patients with new-onset HF reported significantly higher CVD and non-CVD hospitalization and deaths in patients with T2D or CKD than in those without, and were the worst in those with both comorbidities [12]. The high premature morbidity and mortality and associated economic costs associated with CRM conditions heralds an urgent need for developing effective strategies to reduce the burden of complications.
T2D is also associated with a significant economic burden, with estimated costs in the US > $327 billion in 2017, with $237 billion in direct medical costs and $90 billion in reduced productivity due to unemployment from chronic disability and premature mortality [13]. The mean annual direct cost of T2D per person ranges from US $220–7600 in economically developed countries [14]. The cost of treating comorbid CVD in patients with T2D may account for 20–49% of the total amount of T2D care [13, 15]. In patients with T2D and comorbid CKD, the medical costs of diabetes increased significantly with CKD progression, with late-stage CKD being the largest driver [16]. Overall, these findings highlight the significant economic burden associated with comorbid T2D and CKD or CVD.
To our knowledge, a systematic review of the literature on the burden of CRM conditions has not been published previously. Considering the significant clinical and economic burden of CRM conditions, a comprehensive assessment of their impact on economic and humanistic outcomes is needed. This is an important first step in developing holistic management strategies to reduce the burden of CRM conditions to improve quality of life for patients and quality of care and cost efficiencies across healthcare systems. The objective of this systematic literature review (SLR) was to assess the humanistic and economic burden of CRM conditions in patients with CKD, T2D, and CVD.
Methods
Search Strategy and Selection Criteria
This SLR followed the Cochrane Methodology [17], Centre for Reviews and Dissemination [18], and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [19]. PRISMA protocol guidelines were used to prepare the SLR study protocol [20]. Pre-specified inclusion and exclusion criteria were formulated according to the PICOS (population, intervention, comparator, outcome, study type) framework.
The population of interest was individuals ≥ 18 years with CKD, T2D, and CVD. Observational studies, real-world evidence, and economic model studies, with any intervention/comparator, published in English between 2011 and January 10, 2022, as well as abstracts from conferences published from 2020 to 2022, were included. Outcomes of interest included the humanistic burden (health-related quality of life, functional impairment, disability-adjusted life years, quality-adjusted life years, productivity, caregiver burden) and economic burden (direct cost, indirect cost, healthcare resource utilization [HCRU]). When available stage of CKD was identified by stage, typically as follows: Stage 1—estimated glomerular filtration rate (eGFR) ≥ 90 ml/min/1.73 m2 with urine albumin-to-creatinine ratio (UACR) greater than 30 mg/g; Stage 2—eGFR 60–89 ml/min/1.73 m2 with UACR greater than 30 mg/g; Stage 3—eGFR > 30–59 ml/min/1.73 m2; Stage 4—eGFR 15–29 ml/min/1.73 m2; and Stage 5—eGFR < 15 ml/min/1.73 m2.
Studies in pediatric populations not including all three CRM conditions, studies assessing outcomes in response to interventions, or publications primarily focused on validating a tool, were excluded. Studies not originally published in English, studies published pre-2011, and conference abstracts published pre-2020, were also excluded. Details of the rigorous and objective search strategy of the Embase® and Medline® databases are in supplementary eTable S1 and eTable S2. Grey literature searches for additional conference abstract were also performed: from the American College of Cardiology (ACC, 2021–2022), American Heart Association (AHA, 2020–2021), American Society of Nephrology (ASN, 2020–2021), and American Diabetes Association (ADA, 2021–2022).
Study Screening, Quality Assessment, and Data Extraction
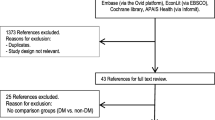
The identification process of studies included in the SLR are presented in a PRISMA flow diagram (supplementary eFigure S1). After removal of duplicates, the papers (title and abstract) were screened for eligibility by one reviewer, and a quality check of 20% of all screened studies was performed by a second reviewer. Full-text articles were obtained for references that met the inclusion criteria, and a second round of eligibility screening was performed, during which the second reviewer quality-checked all screened studies. Any discrepancies during each round were resolved, with arbitration by a third (senior) reviewer. In addition, a manual search of reference lists from relevant publications selected for inclusion was conducted to identify papers missed by the database searches. As before, eligibility screening was performed by one reviewer, and accuracy was validated by a second reviewer.
Observational longitudinal or prospective studies were assessed for risk of bias using the Newcastle–Ottawa Scale (NOS) for cohort studies, while quality assessment of cross-sectional studies used the modified NOS. Full details are in supplementary eTable S3 and eTable S4. The data for relevant outcomes were extracted into a pre-specified Microsoft Excel data-extraction grid by one reviewer, and a second reviewer validated all entries. Race/ethnicity data were collected from the literature, which are typically self-reported. For this article, reference to ethnicity to describe groups can equally be considered as racial groups. Multiple publications reporting the same populations were linked to avoid duplication.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Identification
Of the 1804 records identified, 410 duplicates and 324 irrelevant records were removed. After screening the remaining 1070 records, 980 were excluded. Full-text reports were retrieved for 90 records, of which 73 were excluded, leaving 17 studies. Reasons for study exclusion are in supplementary eTable S5. One additional study, identified from Internet searching, was subsequently included, plus five studies presented as congress abstracts (one of which was superseded by a full-text publication), giving a total of 22 studies (see supplementary eFigure S1).
Study and Patient Characteristics
Details of the 22 studies are presented in Table 1. Of these, 17 (77%) were retrospective cohort studies; with one cross-sectional; one prospective cohort; one budget impact model; one Markov model; and one randomized controlled trial. A total of 14 (64%) studies were conducted in North America. Two publications [21, 22] reported different outcomes from the same population. Baseline characteristics of the participants are presented in supplementary eTable S6. The majority were aged ≥ 65 years. The US and European studies predominantly involved participants identified as having White ethnicity.
Risk of Bias
Studies were scored on a 9-point NOS for quality, where < 5 points suggests poor quality with a high risk of bias, and higher scores suggest a low risk of bias. Low risk of bias was noted in 11/17 (65%) observational studies (8–9 points), whereas 6/17 (35%) observational studies and 1/1 cross-sectional study scored 6–7 indicating medium risk of bias (Table 2).
Study Outcomes
Economic burden in the CRM population was reported in 21/22 studies. Six studies reported cost and HCRU data, seven reported only cost data, and eight reported only HCRU data. One study reported quality-of-life data [23].
Cost and HCRU
Details of the 13 studies containing cost data are shown in supplementary eTable S7, and the 14 studies reporting HCRU data are shown in supplementary eTable S8. As shown in Fig. 1A, B, ten of the cost studies were conducted in the US [22, 24,25,26,27,28,29,30,31,32], with one from Canada [33], Hong Kong [34], and Malaysia [35]. No European studies reported cost data; 9/10 US studies were retrospective cohort studies, with one Markov model study [31]. Similarly, eight of the HCRU studies were conducted in the US [21, 22, 27, 29,30,31, 36, 37] with one from Canada [33]; four were from Europe (one from the UK [12] and Spain [38], and two from Sweden [39, 40]); and one study involved multiple countries across Europe, Australia, and Asia [41] (Fig. 1B).
Geographic breakdown of studies. A All included studies (n = 22). B Studies with assessed outcome, cost (n = 13), HCRU (n = 14), both (n = 21). Abbreviation: HCRU healthcare resource utilization. *Multinational includes Argentina, Australia, Belgium, Bulgaria, Croatia, Germany, Hungary, Israel, Mexico, Netherlands, Poland, Russian Federation, Serbia, Slovakia, Taiwan
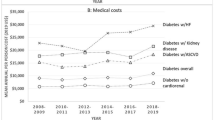
Multimorbidity and advanced CKD were associated with higher medical costs, disease burden, and HCRU for patients with CRM across all studies. Indeed, a Markov model estimated that in 2021 the prevalence of CKD or HF was associated with disproportionally higher expenditure (Fig. 2). Folkerts et al. used the Clinformatics Data Mart database to identify 106,369 patients with T2D and newly diagnosed CKD, and reported a higher prevalence of cardiovascular comorbidities and average annual cost of $24,029/person in the first year post-diagnosis [22]. Patients with comorbid HF and anemia incurred average annual costs of $41,951 and $31,127, respectively; costs were higher compared with patients without these comorbidities. Patients with stage 5 CKD incurred an average cost of $110,210 annually and had an approximate sevenfold-higher annual inpatient hospitalization rate compared with patients with stage 1 CKD.
The relationship between disease prevalence and cost in 2021. Abbreviations: CKD chronic kidney disease, HF, heart failure; T2DM, type 2 diabetes mellitus. Reprinted with permission from the Journal of Managed Care & Specialty Pharmacy: Phil McEwan, Angharad R Morgan, Rebecca Boyce, Natalie Green, Bruce Song, Joanna Huang, Klas Bergenheim, ‘Cardiorenal disease in the United States: Future health care burden and potential impact of novel therapies’, 2022;28(4):415–24. This figure is copyright protected and permission is required from Academy of Managed Care Pharmacy for any reprinting or reuse
Utilizing the same database, Betts et al. analyzed medical costs associated with certain conditions including: the management of CKD, renal replacement therapies (RRTs), and major associated CKD complications including stroke and hyperkalemia. These data were described in a sample size of 52,599 patients with CKD and T2D. Based on their analysis, as disease severity increased, there was an associated higher level of costs and substantially higher RRT. Moreover, CKD management expenditures, when analyzed over approximately 4 months, varied from $7725 for stage 1–2 to a higher level with stage 5 (without RRT) of $11,879. There were even higher additional costs in patients with end-stage renal disease including those on dialysis and kidney transplantation ($87,538 and $124,271, respectively). The subjects incurring or experiencing acute events and major complications had even higher costs, which is consistent with data from previous investigations. For instance, acute event costs for myocardial infarction were $21,016, and $21,087 for stroke, and $31,063 for HF. Within 4 months after the event, it was also noted that these major costs substantially decreased in subsequent 4-month cycles. Other associated cardiovascular events, when experienced acutely, had associated higher costs including $30,500 for atrial fibrillation with hospitalization, and $5162 without. Overall, there were high costs for both CVD and renal-related death. Finally, in the month prior to the terminal event, the associated costs were: CV death, $17,031, renal-related death, $12,605, and due to other causes, $9900. Similar findings of increased costs with CRM and higher hospitalization rates and length of stay with CKD or HF were found across multiple studies, as detailed in Supplementary eTables S7 and S8. These findings are most likely generalizable only to insured patients in the US, and not to other populations.
Interestingly, Clements et al. reported that for T2D alone, patients identified as having Black ethnicity (14.5% of study population) had decreased log odds of receiving any health service use compared to patients identified as having White ethnicity (82.0% of study population). In general, with more chronic conditions, disparities between patients identified as having Black and White ethnicity regarding the probability of any service use decreased, except for patients identified as having Black ethnicity with T2D and stroke, for whom it increased. Disparity also increased slightly for patients identified as having Black ethnicity with T2D and congestive heart failure (CHF)/CKD (beta [standard error (SE)] = – 0.02 [0.03]) but decreased for patients identified as having Black ethnicity with T2D and CHF/chronic obstructive pulmonary disease (COPD)/CKD or with T2D plus CHF/COPD/stroke/CKD [37]. Service-level gaps (assessed as Prevention, Screening, and Monitoring Service use [PMSU]) for different patient groups are presented in Fig. 3. When compared to patients identified as having White ethnicity, there was significantly lower mean service use among those with T2D alone and also in patients identified as having Black, Asian/Pacific Islander, and Hispanic ethnicity with any record of PMSU [37]. Nevertheless, after determining race, ethnicity, and interactions with chronic conditions, this gap was noted to change positively. Specifically, in patients with T2D and certain comorbid conditions, such as CHF/COPD/stroke and CKD, there was a positive change and mean PMSU for patients identified as having Black, Asian/Pacific Islander, and Hispanic ethnicity [37]. This positive change was noted to have significant net differences. These results suggest that patients identified as having White ethnicity with T2D and CHF/COPD/stroke/CKD when, comparing mean level of PMSU relative to other populations and adjusted for covariance, had significantly lower levels. In general, there are several potential reasons why patients identified as having Black ethnicity were noted to have lower odds of using services. In addition, the use of services was higher in patients identified as having White ethnicity when accounting for similar multiple chronic conditions. The potential reasons for these differences include a lack of awareness of the need for routine screening among the population of patients identified as having Black ethnicity with other multiple comorbidities and a potential overall diminished trust in the health care system. This limited trust is based on multiple factors including previous lived experiences and decreased motivation to seek treatment. Therefore, when patients identified as having Black ethnicity do access treatment, the level of morbidity may have increased, requiring more resources to treat multiple conditions compared to similarly described patients identified as having White ethnicity [37].

Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association
Service-level gaps for patients from racial/ethnic minority populations compared with patients identified as having White ethnicity. Negative values indicate PMSU levels that are less than those for patients identified as having White ethnicity, while positive values represent PMSU levels that are more than those for patients identified as having White ethnicity. Abbreviation: AIAN American Indian/Alaska Native, A/PI Asian/Pacific Islander, CHF congestive heart failure, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, MCC multiple chronic condition, SE standard error. Reproduced from ‘Race Disparities in the Use of Prevention, Screening, and Monitoring Services in Michigan Medicare Beneficiaries With Type 2 Diabetes and Combinations of Multiple Chronic Conditions’, Clements et al., Clin Diabetes, 2020.
Quality of Life
Only one study reported data on the humanistic burden of patients with CRM. Wang et al. assessed the depressive symptoms of CKD in China in 210 patients with T2D in a cross-sectional study using the Hospital Anxiety and Depression Scale (HAD-D) [23]. The mean age of participants was 57.6 years, and over 20% (45/210) of participants reported depressive symptoms, with female gender (P = 0.010), hypertension (P = 0.022), stages 4 (P = 0.003) and 5 (P < 0.001) CKD (i.e., severity of T2D-related CKD) being statistically significant risk factors for depressive symptoms. Participants with HAD-D score < 11 had a significantly better quality of life than those with HAD-D score ≥ 11.
Discussion
This SLR aimed to assess the economic and humanistic burden of CRM conditions for patients with T2D, CKD, and CVD. To our knowledge, this is the first SLR on this topic. Of the 22 studies, the majority were observational retrospective cohort studies conducted in the US of patients aged ≥ 65 years. While research into CRM conditions is limited, the available evidence points to higher costs and HCRU in patients with all three conditions compared to those with two or no comorbidities. In addition, increasing age was associated with a higher number of comorbidities, indicating that older patients are a particularly vulnerable population.
Higher healthcare expenditure and service utilization were observed among urban insured patients than in the uninsured and racial/ethnic minority populations [25]. Patients identified as having Black ethnicity also had lower odds of using healthcare services compared to patients identified as having White, Asian/Pacific Islander, and Hispanic ethnicity [37]. These disparities in access to care may be due to a lack of disease awareness and trust in the healthcare system, and structural inequalities in healthcare delivery, as the US healthcare system better serves those with financial resources/comprehensive health insurance, than the poor or uninsured/underinsured [25, 37, 42]. However, patients identified as having Black ethnicity with multiple comorbidities tended to have higher service utilization, which was possibly related to reduced service use early in their clinical course leading to more severe disease later.
Dieleman and colleagues reported a disproportionately large amount of US healthcare spending on individuals identified as having White ethnicity compared to other racial/ethnic backgrounds, which increased over time. This included higher spending on ambulatory and primary care, which plays a crucial role in preventive healthcare. In contrast, individuals identified as having Black ethnicity had higher spending on emergency, inpatient, and nursing facility care. This difference in healthcare utilization suggests that individuals identified as having Black ethnicity may lack access to ambulatory care, which serves a critical preventive role. These spending differences could not be explained by the numbers in each race/ethnicity group diagnosed with a health condition. This SLR supports the existing literature on downstream factors that perpetuate healthcare inequality by race and ethnicity, such as responses of physicians to patients, residential segregation preventing easy access to healthcare services, and lack of diversity in the healthcare workforce reinforcing mistrust [42,43,44].
Inpatient hospital admissions were the highest source of increased costs in patients with CRM followed by outpatient visits, pharmacy costs, and visits to the emergency room. These costs are largely driven by a lack of quality preventive care and the absence of a universal healthcare system. Diagnoses of CKD and HF were associated with increased hospitalizations and longer lengths of stay. CKD-related complications, worsening CKD severity, and progression to RRT were associated with increased costs. Several large retrospective cohort studies showed that costs and HCRU were higher with increasing CKD stages [12, 22, 27, 40]. In the study by Folkerts et al. [22], patients with comorbid HF and anemia incurred higher costs than patients without these comorbidities, with increased costs in the first year after diagnosis of CKD. The risk of HF hospitalization increased with deteriorating renal function in Swedish patients with T2D [40]. Higher CVD hospitalization rates occurred in the first year in UK patients with HF, T2D, and CKD compared to patients with only CKD/T2D [12]. Furthermore, these patients had higher hospitalization rates and longer lengths of stay. Blankenburg et al. 2020 [27] also reported increased visits to the emergency department by patients with CKD. As expected, in addition to higher costs with increasing CKD stages, costs were substantially higher with RRT [28].
In regard to the humanistic burden in patients with CRM, a single retrospective observational study from China showed that particularly female patients with late-stage disease, T2D, CKD, and hypertension, were at higher odds of experiencing depressive symptoms compared with patients without hypertension [23].
This SLR has identified evidence gaps in the published literature. Most evidence comes from studies of the insured US population, while research of the non-insured and ethnic minority populations is lacking. Further, there is a scarcity of evidence from racial/ethnic groups other than the population of patients identified as having White ethnicity and data on European healthcare costs that needs to be addressed. Additionally, the humanistic burden in patients with CRM is severely understudied with only a single report found. A key strength of this review is its systematic approach and quality assessment, with study selection and quality assessment performed independently by two researchers. While limited research exists, the available evidence shows a clear trend of increasing costs and HCRU with increasing number of CRM comorbidities.
Limitations of this SLR include that the studies identified did not cover the COVID-19 pandemic period and therefore the impact of COVID-19 burdens and/or restricted access to healthcare during the pandemic. Also, there may be selection and publication bias, as only English language studies were included. This SLR may have omitted relevant publications not indexed in the Embase or Medline databases, or not identified in the hand searches of ACC, AHA, ASN, or ADA congress abstracts, as well as any published outside of the search period (January 1, 2011—January 10, 2022 for databases or 2020–2022 for congress abstracts). Most studies were retrospective while only two had a prospective design. There may be data in publications that were relevant but not the primary outcome and therefore the title and abstract did not allow for capturing in the search process. The results are difficult to generalize and comparisons between groups based on social status, race/ethnic group, and geography are complicated. Lastly, the healthcare costs are not adjusted for inflation or national standard of living, so cannot be compared over time or between countries. Despite this, the consistency of trends identified in this SLR clearly indicate a higher economic burden associated with CRM conditions.
Conclusions
This SLR found a clear trend of increasing healthcare costs and resource use with an increasing number of comorbidities, indicating a greater economic burden for patients with CRM conditions. This review also identified a higher number of comorbidities with increasing age leading to greater economic burden among patients ≥ 65 years. Given the sparsity of data from Europe and American populations other than individuals identified as having White ethnicity and insured, further research is needed to evaluate the economic and humanistic burden of CRM conditions in these lesser studied marginalized and higher risk groups. Targeted interventions are also needed in patients with CRM multimorbidities to reduce healthcare costs and improve outcomes, such as early and more aggressive lifestyle and evidenced-based pharmacologic interventions.
Data availability
All data associated with this SLR are in the public domian.
References
International Diabetes Federation. IDF Diabetes Atlas, 10th edition. 2021. https://diabetesatlas.org/. Accessed 14 Sep 2022.
ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S158–90.
Ceriello A, Catrinoiu D, Chandramouli C, et al. Heart failure in type 2 diabetes: current perspectives on screening, diagnosis and management. Cardiovasc Diabetol. 2021;20(1):218.
Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–18.
Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(5):853–72.
Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12(2):73–81.
Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316(6):602–10.
Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37(10):2864–83.
Kadowaki T, Maegawa H, Watada H, et al. Interconnection between cardiovascular, renal and metabolic disorders: a narrative review with a focus on Japan. Diabetes Obes Metab. 2022;24(12):2283–96.
Arnold SV, Kosiborod M, Wang J, Fenici P, Gannedahl G, LoCasale RJ. Burden of cardio-renal-metabolic conditions in adults with type 2 diabetes within the Diabetes Collaborative Registry. Diabetes Obes Metab. 2018;20(8):2000–3.
Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–8.
Lawson CA, Seidu S, Zaccardi F, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. EClinicalMedicine. 2021;32: 100739.
American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28.
Ramzan S, Timmins P, Hasan SS, Babar ZU. Cost analysis of type 2 diabetes mellitus treatment in economically developed countries. Expert Rev Pharmacoecon Outcomes Res. 2019;19(1):5–14.
Einarson TR, Acs A, Ludwig C, Panton UH. Economic burden of cardiovascular disease in type 2 diabetes: a systematic review. Value Health. 2018;21(7):881–90.
Slabaugh SL, Curtis BH, Clore G, Fu H, Schuster DP. Factors associated with increased healthcare costs in Medicare Advantage patients with type 2 diabetes enrolled in a large representative health insurance plan in the US. J Med Econ. 2015;18(2):106–12.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021.
Tacconelli E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect Dis. 2010;10(4):226.
Page MJ, McKenzie J, Bossuyt P. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2020:1–36.
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350: g7647.
Folkerts K, Kelly AMB, Petruski-Ivleva N, et al. Cardiovascular and renal outcomes in patients with type-2 diabetes and chronic kidney disease identified in a united states administrative claims database: a population cohort study. Nephron. 2021;145(4):342–52.
Folkerts K, Petruski-Ivleva N, Kelly A, et al. Annual health care resource utilization and cost among type 2 diabetes patients with newly recognized chronic kidney disease within a large U.S. administrative claims database. J Manag Care Spec Pharm. 2020;26(12):1506–16.
Wang X, Shen B, Zhuang X, Wang X, Weng W. Investigating factors associated with depressive symptoms of chronic kidney diseases in China with Type 2 diabetes. J Diabetes Res. 2017;2017:1769897.
Nichols GA, Vupputuri S, Lau H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care. 2011;34(11):2374–8.
Ozieh MN, Dismuke CE, Lynch CP, Egede LE. Medical care expenditures associated with chronic kidney disease in adults with diabetes: United States 2011. Diabetes Res Clin Pract. 2015;109(1):185–90.
Blankenburg M, Fett AK, Eisenring S, Haas G, Gay A. Patient characteristics and initiation of mineralocorticoid receptor antagonists in patients with chronic kidney disease in routine clinical practice in the US: a retrospective cohort study. BMC Nephrol. 2019;20(1):171.
Blankenburg M, Kovesdy CP, Fett AK, Griner RG, Gay A. Disease characteristics and outcomes in patients with chronic kidney disease and type 2 diabetes: a matched cohort study of spironolactone users and non-users. BMC Nephrol. 2020;21(1):61.
Betts KA, Song J, Faust E, et al. Medical costs for managing chronic kidney disease and related complications in patients with chronic kidney disease and type 2 diabetes. Am J Manag Care. 2021;27(20 Suppl):S369–74.
Li Y, Barve K, Cockrell MM, Agarwal A, Casebeer AW, Poonawalla IB. Well-managed CKD and its association with healthcare resource utilization and costs. American Society of Nephrology. 2021.
Olufade T, Jiang L, Israni R, Huang J, Gosmanov AR. Cardiovascular and renal disease manifestation and healthcare resource utilization in patients on first-line oral therapy for type 2 diabetes: a claims-based observational cohort study. Diabetes Obes Metab. 2021;23(12):2741–51.
McEwan P, Morgan AR, Boyce R, et al. Cardiorenal disease in the United States: future health care burden and potential impact of novel therapies. J Manag Care Spec Pharm. 2022;28(4):415–24.
Nichols GA, Qiao Q, Linden S, Kraus B. Medical costs among incident heart failure patients with reduced, mildly reduced, and preserved ejection fraction. J Am Coll Cardiol. 2022;79(9 Suppl.):283.
Rapattoni W, Zante D, Tomas M, et al. A retrospective observational population-based study to assess the prevalence and burden of illness of type 2 diabetes with an estimated glomerular filtration rate < 90 ml/min/1.73 m(2) in Ontario, Canada. Diabetes Obes Metab. 2021;23(4):916–28.
Wan EYF, Chin WY, Yu EYT, et al. The impact of cardiovascular disease and chronic kidney disease on life expectancy and direct medical cost in a 10-year diabetes cohort study. Diabetes Care. 2020;43(8):1750–8.
Mohd-Tahir NA, Li SC. Budget impact analysis of increasing prescription of renin-angiotensin system inhibitors drugs to standard anti-hypertensive treatments in patients with diabetes and hypertension in a hypothetical cohort of Malaysian population. PLoS ONE. 2019;14(2): e0212832.
Yandrapalli S, Malik A, Namratha F, et al. Impact of diabetes mellitus and its interactions with other cardiovascular risk factors on heart failure hospitalizations following acute myocardial infarction. J Am Coll Cardiol. 2021;77(18 Suppl. 1):1617.
Clements JM, West BT, Harissa B, Hayden N, Khan MM, Palepu R. Race disparities in the use of prevention, screening, and monitoring services in Michigan Medicare beneficiaries with type 2 diabetes and combinations of multiple chronic conditions. Clin Diabetes. 2020;38(4):363–70.
Arevalo-Lorido JC, Carretero-Gomez J, Aramburu-Bodas O, Grau-Amoros J, Torres-Cortada G, Camafort-Babkowski M. Blood pressure, congestion and heart failure with preserved ejection fraction among patients with and without type 2 diabetes mellitus. A cluster analysis approach from the observational registry DICUMAP. High Blood Press Cardiovasc Prev. 2020;27(5):399–408.
Rosengren A, Edqvist J, Rawshani A, et al. Excess risk of hospitalisation for heart failure among people with type 2 diabetes. Diabetologia. 2018;61(11):2300–9.
Tancredi M, Rosengren A, Olsson M, et al. The relationship between three eGFR formulas and hospitalization for heart failure in 54 486 individuals with type 2 diabetes. Diabetes Metab Res Rev. 2016;32(7):730–5.
Kalantar-Zadeh K, Schwartz GG, Buhr KA. Effect of Apabetalone on Major Adverse Cardiovascular Events in Patients with CKD, Diabetes, and Recent Acute Coronary Syndrome: Results from the BETonMACE Trial. American Society of Nephrology. 2020.
Dieleman JL, Chen C, Crosby SW, et al. US health care spending by race and ethnicity, 2002–2016. JAMA. 2021;326(7):649–59.
Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med. 2018;199:219–29.
Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447–53.
Acknowledgements
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors received no payment related to the development of the manuscript.
Medical Writing and Editorial Assistance
Writing support was provided by Debra Brocksmith, MB ChB, PhD, and Céline Tevlin, PhD, of Envision Pharma Group, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI) and Lilly, USA LLC. BIPI and Lilly were given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The systematic literature review was conducted by Curo, a division of Envision Pharma Group, which was contracted and funded by BIPI and Lilly. The authors wish to thank Jatin Gupta, MPharm, and Karen Smoyer, PhD, for assistance with the systematic literature review.
Funding
This systematic literature research and the journal’s Rapid Service Fee was funded by Boehringer Ingelheim Pharmaceuticals Inc. and Lilly, USA LLC.
Author information
Authors and Affiliations
Contributions
Keith Ferdinand, Keith C. Norris, Helena W. Rodbard, and Jennifer M. Trujillo contributed to the scope and search terms of the systematic literature research, analysis, or interpretation of data and development of the manuscript. All authors had full access to all the search findings and had final responsibility for the decision to submit for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
Keith C. Ferdinand has served as a consultant for Amgen, Boehringer Ingelheim, Eli Lilly and Company, Janssen, and Medtronic. Keith C. Norris has served as a consultant for Atlantis Health Care for quality improvement, and is supported in part by NIH grants UL1TR000124, P30AG021684, and P50MD017366. Helena W. Rodbard has served as a consultant for, and received research support from, Eli Lilly, Novo Nordisk, Sanofi, Medtronic, Merck, Pacira, and Regeneron. Jennifer M. Trujillo has served as a consultant for Novo Nordisk, and Sanofi.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ferdinand, K.C., Norris, K.C., Rodbard, H.W. et al. Humanistic and Economic Burden of Patients with Cardiorenal Metabolic Conditions: A Systematic Review. Diabetes Ther 14, 1979–1996 (2023). https://doi.org/10.1007/s13300-023-01464-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01464-8