Abstract
Introduction
Left ventricular global longitudinal strain (GLS) is considered to be the first marker of diabetes mellitus-related subclinical cardiac dysfunction, but whether it is attributable to fat mass and distribution remains uncertain. In this study, we explored whether fat mass, especially fat mass in the android area, is associated with subclinical systolic dysfunction before the onset of cardiac disease.
Methods
We conducted a single-center prospective cross-sectional study between November 2021 and August 2022 on inpatients of the Department of Endocrinology, Nanjing Drum Tower Hospital. We included 150 patients aged 18–70 years with no signs, symptoms, or history of clinical cardiac disease. Patients were evaluated with speckle tracking echocardiography and dual energy X-ray absorptiometry. The cutoff values for subclinical systolic dysfunction were set at a global longitudinal strain (GLS) < 18%.
Results
After adjusting for sex and age, patients with GLS < 18% had a higher mean (± standard deviation) fat mass index (8.06 ± 2.39 vs. 7.10 ± 2.09 kg/m2, p = 0.02), higher mean trunk fat mass (14.9 ± 4.9 vs. 12.8 ± 4.3 kg, p = 0.01), and higher android fat mass (2.57 ± 1.02 vs. 2.18 ± 0.86 kg, p = 0.02) than those in the GLS ≥ 18%. Partial correlation analysis showed that the fat mass index, truck fat mass, and android fat mass were negatively correlated with GLS after adjusting for sex and age (all p < 0.05). Adjusted for traditional cardiovascular metabolic factors, fat mass index (odds ratio [OR] 1.27, 95% confidence interval [CI] 1.05–1.55, p = 0.02), trunk fat mass (OR 1.13, 95% CI 1.03–1.24, p = 0.01), and android fat mass (OR 1.77, 95% CI 1.16–2.82, p = 0.01) were independent risk factors for GLS < 18%.
Conclusion
Among patients with type 2 diabetes mellitus without established clinical cardiac disease, fat mass, especially android fat mass, was associated with subclinical systolic dysfunction independently of age and sex.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with diabetes mellitus have an increased risk of heart failure compared to the people without diabetes mellitus. |
To prevent heart failure, early screening of cardiac function and early intervention are essential. |
Diabetes mellitus-related subclinical cardiac dysfunction can be detected by left ventricular global longitudinal strain (GLS), a marker of systolic function, but it is unclear whether the GLS is related to fat mass and distribution. |
What was learned from the study? |
Subclinical left ventricular systolic dysfunction is closely linked to fat mass, especially android fat mass. |
The results of this study indicate that fat mass may be a potential target in the treatment of patients with type 2 diabetes mellitus without overt cardiac disease. |
Introduction
Cardiovascular disease (CVD) is the leading cause of death in patients with diabetes mellitus [1]. The major CVDs mostly commonly found in patients with diabetes mellitus are atherosclerotic cardiovascular disease (ASCVD) and heart failure. Compared to people without diabetes mellitus, patients with diabetes mellitus have a twofold increased risk of heart failure [2]. Moreover, heart failure can present as heart failure with preserved ejection fraction (HFpEF) and as heart failure with reduced ejection fraction (HFrEF) [3].
Early screening of cardiac function and early intervention are essential to prevent heart failure [4]. Before heart failure becomes apparent, subclinical changes in cardiac function occur in individuals with diabetes mellitus [5]. Patients with a higher risk of heart failure can be identified using novel speckle tracking ultrasound technology [6]. A reliable marker of systolic function is global longitudinal strain (GLS), which can be measured by speckle tracking ultrasound. The accuracy of strain assessment has been widely validated [7, 8]. Before the onset of clinical symptoms of heart failure, left ventricular dysfunction in patients with diabetes mellitus is characterized by impaired GLS, impaired diastolic function, or both [5]. Impaired GLS is considered to be an early imaging manifestation of diabetic cardiomyopathy [9]. Thus, assessment of longitudinal strain with the speckle tracking ultrasound technique allows early screening for altered cardiac function.
For early management, it is critical that both high-risk populations and risk factors are identified and interventions initiated. Epidemiologic studies of heart failure suggest that the Asian patient population has a distinct HFpEF phenotype, with a relatively lower body mass index (BMI) but a higher prevalence of diabetes mellitus. This phenotype also has the most adverse prognosis of all heart failure phenotypes [10]. Also, compared to people with diabetes mellitus in the Western population, obesity in people in the Asian population with diabetes mellitus is characterized by a lower BMI with central adiposity [11]. These studies emphasize that in Asian patient populations with diabetes mellitus, changes in body composition and distribution are more important for disease prevention than BMI alone. In recent years, indicators of body composition or distribution other than BMI have been shown to have better discriminatory power than BMI in predicting cardiovascular outcomes [12, 13].
However, whether region-specific fat can be a controllable risk factor for early cardiac impairment and a potential target for early intervention is unclear. Previous studies have attempted to explore the relationship between adiposity and early changes in cardiac function, but have not focused specifically on abdominal fat [14]. In patients with abdominal obesity, dual-energy X-ray absorptiometry (DEXA) can be used to assess abdominal fat mass, which contributes to CVD. However, it has not been widely used in relevant studies [15].
Therefore, in the present study we used DEXA to measure fat mass, with a focus on fat in the android region. We also investigated whether fat mass is associated with subclinical systolic dysfunction before the onset of heart disease.
Methods
Study Population
For this study, patients were prospectively recruited from November 2021 to August 2022 in the inpatient setting of the Department of Endocrinology, Nanjing Drum Tower Hospital. Patients were eligible if they were aged 18–70 years, diagnosed with type 2 diabetes mellitus (T2DM), had no established cardiovascular complications, and were willing to participate. Established CVD was defined as heart failure (stage 3–4); previous myocardial infarction, stable angina, previous percutaneous coronary intervention, or coronary artery bypass surgery; atrial fibrillation; and/or congenital heart disease. Exclusion criteria were: (1) diagnosis of other types of diabetes mellitus; (2) diabetic ketosis; (3) T2DM combined with endocrine disease, including diagnosed pituitary adenomas, Cushing syndrome, primary hyperaldosteronism, pheochromocytoma, hypothyroidism, and hyperthyroidism; (4) malignant tumors; (5) anemia; (6) infections requiring antibiotic treatment; (7) left ventricular ejection fraction (LVEF) < 50%; (8) suboptimal image quality; and (9) refusal to undergo speckle tracking echocardiography or DEXA. The flowchart of the study is shown in Fig. 1.
The study was performed in accordance with the Helsinki Declaration of 1964 and its subsequent amendments. Ethical approval was provided by the Nanjing Drum Tower Hospital Ethics Committee (No. 2021-573-01). All participants provided written informed consent to participate.
General Examinations
Endocrinologists collected demographic information, medical history, and anthropometric measurements. Blood pressure measurements were performed on the same day as the echocardiography measurements. Hypertension was determined by pressure measurements or on previous medical history. Biochemical profiles were determined from a fasting blood sample. The standard meal test (with 75 g of carbohydrate) was used to assess plasma glucose levels. Endocrinologists evaluated patients for microvascular complications, including diabetic kidney disease, diabetic retinopathy, and diabetic peripheral neuropathy. The data on the patient's present medical history and physical examination were collected on the day of admission, while the data on laboratory tests and the evaluation of complications were obtained through blood tests within 72 h after admission. Diagnosis of complications was based on the patient's past medical history and the examination during the current hospitalization.
Dual-Energy X-Ray Absorptiometry
Dual-energy X-ray absorptiometry (GE Healthcare Technologies, West Milwaukee, WI, USA) was used to assess body composition. The total mass, fat mass, and muscle mass were measured using iDXA software (GE Healthcare Technologies). Total body mass measurement, including fat and muscle measurements, were obtained from all body parts (i.e., head, trunk, arms, and legs). The horizontal line of the pelvic inlet was set as the lower edge. From this line upwards, the range of 20% of the trunk length is defined as the android area. Variables of total and regional body composition were defined as follows: (1) fat mass index (FMI) = total fat mass (kg)/(height [m])2 × 100; (2) body surface area (BSA) = (0.61 × height [m]) + (0.0128 × weight [kg]) − 0.1529; (3) android fat mass to height2 = total fat mass (kg)/(height [m])2; and (4) android fat mass to weight = total fat mass (kg)/weight(kg)*100%.
Echocardiography and Left Ventricular Global Longitudinal Strain
One senior physician performed transthoracic echocardiography using a GE Vivid E95 ultrasound system (GE Healthcare Technologies). In accordance with the American Society of Echocardiography's current guidelines [16], the size of each chamber, wall thickness, valve shape, cardiac activity and motion, blood flow, and LVEF were measured. The left ventricular mass index (LVMI) was defined as left ventricular mass indexed to the BSA and calculated according to the guidelines [17]. The LVEF was estimated using the M-mode method. Diastolic function was assessed using color M-mode and tissue Doppler measurements. During end-expiratory apnea, apical four-axis, two-axis, and long-axis views were acquired for offline strain analysis.
One physician analyzed all stored echocardiography images using software applications (EchoPAC version 113; General Electric Medical Systems, GE Healthcare Technologies). The endocardial border was automatically tracked using this software package, and the results of the analysis were accepted after visual inspection when the software indicated adequate tracking or after manual adjustments. The GLS was calculated as the mean peak longitudinal strain over 18 left ventricular segments. The left ventricular longitudinal systolic dysfunction cutoff point was set at an absolute value of GLS < 18%. The bull's-eye views of patients with left ventricular longitudinal systolic dysfunction and of patients without ventricular longitudinal systolic dysfunction are shown in Figs. 2 and 3, respectively.
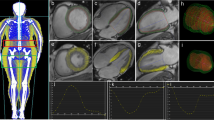
Bull’s-eye views of the peak systolic myocardial longitudinal strain of patients with type 2 diabetes mellitus (T2DM) and subclinical left ventricular systolic dysfunction. Observations of strain values are shown in three different cardiac views: the four-chamber view (4CH), the two-chamber view (2CH), and the apical long-axis view (APLAX) view. Strain curves are plotted as changes in myocardial strain values over one cardiac cycle in the left ventricle. Different colors represent different segments of the myocardium, and the stages of the cardiac cycle are marked by the electrocardiogram under the strain curves. The peak strain values for all segments are summarized as a bull's-eye plot, with each segment's peak strain value indicated by a numerical value within the plot. The average of all segmental peak strain values represents the global longitudinal strain (GS). In this study, the GLS of the left ventricle was 15.0%, which is below the normal cutoff point
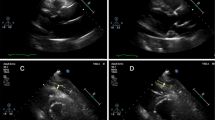
Bull’s-eye views of the peak systolic myocardial longitudinal strain of patients with T2DM but without subclinical left ventricular systolic dysfunction. Observations of strain values in three different cardiac views: 4CH, 2CH, and APLAX. Strain curves are plotted as changes in myocardial strain values over one cardiac cycle in the left ventricle. Different colors represent different segments of the myocardium, and the stages of the cardiac cycle are marked by the electrocardiogram under the strain curves. The peak strain values for all segments are summarized as a bull's-eye plot, with each segment's peak strain value indicated by a numerical value within the plot. The average of all segmental peak strain values represents the global longitudinal strain (GS). In this study, the GS of the left ventricle was 20.8%, which is normal
The intra-observer variability for GLS was evaluated in 15 randomly selected participants, with a minimum of 2 months passed between measurements.
Statistical Analysis
Because both left ventricular systolic dysfunction and body composition differed by sex and age, we applied propensity score matching to adjust for sex and age in our descriptive analysis. We used logistic regression models to estimate propensity scores for each patient based on their age and sex. We then used a nearest neighbor matching algorithm to match patients in the GLS < 18% group (i.e., the subclinical cardiac dysfunction group) with those in the control group (GLS ≥ 18%) based on their propensity scores, without a specific caliper width of the propensity score and without trimming procedure. In this study, continuous variables that were normally distributed are expressed as the mean ± standard deviation, otherwise as the median with interquartile range. The independent sample t-test or the Mann–Whitney test was used to compare groups. Categorical variables are expressed as absolute values with percentages and compared between groups using Chi-square tests. Intra-observer variability for GLS was calculated using an intra-class correlation coefficient (ICC) and 95% confidence interval (CI). Associations between body mass and ultrasound image measurement were assessed by correlation and partial correlation analysis in participants with T2DM. Based on the body mass and composition indicator, we grouped patients into three quartiles. We compared the differences in GLS between the three groups by analysis of variance and a post hoc comparison using the Turkey’s test. Multivariable linear regressions were performed to assess independent associations between body mass measurements and cardiac function. Multivariable logistic regression was used to assessed independent associations between body mass measurements and GLS < 18%. Statistical analysis was performed using the R.4.1.0 software package ® Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered to indicate statistical significance.
Results
Demographic and Biochemical Results in Patients with Subclinical Left Ventricular Systolic Dysfunction
The inclusion and exclusion process for enrolled patients is shown in Fig. 1. We initially recruited 203 patients for clinical evaluation, of whom 161 underwent echocardiographic evaluation. Of these 161 patients, 11 were excluded due to suboptimal ultrasound imaging (n = 6), congenital heart disease (n = 3), or severe to moderate valvular diseases (n = 2), leaving 150 patients to enter the final analysis. Intra-observer variability analysis suggested that GLS has good reproducibility (ICC 0.996, 95% CI 0.987–0.998, p < 0.001).
The general demographics and clinical characteristics of the patients before and after propensity score matching are summarized in Table 1. Of the 150 patients included in the final analysis, 44% were categorized into the subclinical cardiac dysfunction group (GLS < 18% group). After matching for age and sex, patients with GLS < 18% were found to have a higher BMI than those in the control group (GLS ≥ 18% group) (26.5 ± 3.8 vs. 25.1 ± 3.4 kg/m2, p = 0.02). The two groups of patients had similar proportions of hypertension, microvascular complications, antihypertensive and lipid-lowering medication use (all p > 0.05).
Echocardiographic Parameters and Body Compositions of Patients Before and After Propensity Score Matching
As summarized in Table 2, after matching for sex and age, patients in the subclinical cardiac dysfunction group (GLS < 18%) were found to have a higher fat mass index (8.06 ± 2.39 vs. 7.10 ± 2.09 kg/m2, p = 0.02), higher trunk fat mass (14.9 ± 4.9 vs. 12.8 ± 4.3 kg, p = 0.01), and higher android fat mass (2.57 ± 1.02 vs. 2.18 ± 0.86 kg, p = 0.02) than those in the GLS ≥ 18% group. This relationship persisted after dividing the value of android fat mass by the square of height (0.87 ± 0.34 vs. 0.74 ± 0.28 kg/m2, p = 0.02), by weight (3.18 ± 0.82 vs. 2.90 ± 0.76%, p = 0.04), and by body surface area (1.32 ± 0.42 vs. 1.17 ± 0.36 kg/m2, p = 0.02). However, there were no significant differences in muscle composition-related indicators (all p > 0.05). As shown in Fig. 4, grouped by the tertiles of the body fat mass correlation parameters, patients with the highest android fat mass had significantly the lowest GLS.
Box plot comparing global longitudinal strain (GLS) after tertile grouping (T1, T2, T3) based on parameters related to body composition. A GLS in tertiles of android fat mass, B GLS in tertiles of truck fat mass, C GLS in tertiles of fat mass index, D GLS in tertiles of body mass index. Asterisks indicate a significant difference between tertiles at *p < 0.05, **p < 0.01, and ***p < 0.001 (Turkey’s test). NS Not significant
Correlation and Partial Correlation Between GLS and Clinical Parameters
As shown in Table 3, after adjusting for sex and age, GLS was correlated with fat mass index (r = − 0.203, p = 0.014), trunk fat mass (r = − 0.228, p = 0.005), and android fat mass (r = − 0.215, p = 0.009).
Fat Mass is Related to Subclinical Left Ventricular Systolic Dysfunction in Regression Analysis
The results of the regression analysis are shown in Tables 4 and 5. After adjusting for sex, age, glycated hemoglobin, systolic blood pressure, and triglycerides, multivariate linear regression indicated negative associations between GLS and FMI (β = − 0.22, 95% CI − 0.37 to − 0.06, p = 0.01), trunk fat mass (β = − 0.12, 95% CI − 0.19 to − 0.05, p < 0.01), and android fat mass (β = − 0.55, 95% CI − 0.90 to − 0.20, p < 0.01). Multivariate logistic regression showed associations between subclinical systolic dysfunction (GLS < 18%) and FMI (odds ratio [OR] 1.27, 95% CI 1.05–1.55, p = 0.02), trunk fat mass (OR 1.13, 95% CI 1.03–1.24, p = 0.01), and android fat mass (OR 1.77, 95% CI 1.16–2.82, p = 0.01). As shown in Electronic Supplementary Material Fig. 1, the associations still held when each medication class was used as a covariate.
Discussion
The results of our study show that fat mass, especially android fat mass, is associated with impaired subclinical systolic function in patients with T2DM. We found that this relationship still existed when the parameters of android fat were adjusted based on height, weight, and BSA, suggesting that abdominal fat may have been responsible for impaired subclinical systolic function prior to symptoms of heart failure.
In the present study, we performed an early assessment of systolic function in patients without CVD. Subclinical systolic dysfunction appears to be common in patients with diabetes mellitus. Holland et al. reported that 45% of asymptomatic patients had impaired longitudinal strain at baseline, independently associated with adverse outcomes [18]. These results are comparatively consistent our results: 44% of patients enrolled in our study demonstrated decreased subclinical systolic function without CVD and heart failure symptoms. This prevalence is higher than that reported in some studies; for example, Ernande et al. reported impaired longitudinal strain in 32% of patients without overt heart disease and hypertension [19]. Hypertension is a common co-morbidity in patients with diabetes mellitus before the onset CVD. The patients enrolled in our study represent the broader asymptomatic population. The LVEF of patients in similar studies was normal [18, 19]. Our results are consistent with those of these previous studies and revealed that the LVEF was lower in patients with impaired GLS. In evaluations of systolic function, both indicators are consistent, but GLS is more sensitive. With the rise of HFpEF as the predominant phenotype in heart failure, conventional LVEF does not reflect changes in the disease course and, therefore, this parameter seems less advantageous for early detection and continuous monitoring of CVDs [16]. Despite the lack of massive evidence on patient selection, treatment efficacy, and economic impact, screening for subclinical left ventricular dysfunction in metabolic disease enables cardioprotective therapy to be implemented at an early stage to prevent or delay the progression of heart failure [8]. Such screening is expected to be increasingly feasible with miniaturized echocardiography equipment and automated analysis [20, 21].
Weight management is critical for preventing CVD in patients with diabetes mellitus [22]. The findings of our study support the conclusion that patients with high BMI have an increased risk of heart failure in the asymptomatic phase. Similar to our findings, Kishi et al. reported chronic obesity from young adulthood to middle age was associated with impaired left ventricular systolic and diastolic function [23]. In their study on overweight patients, Suto et al. found a higher proportion of impaired strain in Asian patients with diabetes mellitus [24]. These consistent findings highlight the role of weight management in preventing early cardiac function changes.
Although overweight and obesity are associated with CVD, there is a large heterogeneity among patients. BMI is a single indicator used to evaluate obesity, but it does not reflect the amount and distribution of fat and muscle. Over the recent decades, many studies have reported links between body composition and heart failure [25, 26]. However, whether this effect is already present in the preclinical phase of heart failure is unclear. Hatani et al. explored the association of GLS with fat mass index measured by bioelectrical impedance analysis (BIA) [14]. Our study included a similar population, but had a larger sample size. We used a more accurate method of assessing fat mass by DEXA and found that this correlation still holds. The study of Hatani et al. did not distinguish between abdominal adipose tissue due to the limitations of the BIA [14]. We further identified regional fat mass and suggested that trunk fat and android fat are also similarly associated. Central obesity is assumed to be more closely related to CVD. Previous studies usually defined central obesity in terms of waist circumference. Selvaraj et al. found that patients with central adiposity had adverse cardiac mechanics, based on assessments of central obesity by waist circumference and waist-to-hip ratio only [27]. However, the waist measurement is not able to distinguish between abdominal fat and muscle. In our study, fat mass in the android region—and not muscle mass—was a risk factor for subclinical systolic dysfunction. The results of our study and of previous studies suggest that increased body fat, especially abdominal fat, is a risk factor for subclinical systolic dysfunction, also in patients with diabetes mellitus.
The composition of the body changes with age, with the more elderly population having less muscle and more fat, especially visceral fat. The authors of the STRAMBO study reported that elderly men had an increased gynoid fat mass index and android fat mass index than did young adult men [28]. Previous studies on body composition and cardiac function have been conducted mostly in the elderly, who are more susceptible to sarcopenia. On one study in healthy 70-year-old males, cardiac function was found to be correlated with lean body mass [29]. In older general populations, Garg et al. found central obesity to be very robustly associated with impaired left ventricular systolic strain [30]. Our study, however, focused on patients with diabetes mellitus, which included a relatively young population; thus, we extended the finding to another high-risk population for HFpEF. In our study, considering that middle-aged patients are less affected by sarcopenia, muscle content was slightly increased in the group with impaired cardiac function, but the difference was not statistically significant. It is noteworthy that the percentage of muscle mass to body weight was lower in this group. As a result, fat appears to play a more important role in cardiac function than muscle. Therefore, the control of body fat content in this population should be emphasized. Fat mass and distribution differ significantly between men and women. Ley and colleagues reported that the proportion of android fat was significantly higher in men [31]. Results from previous studies also suggested that GLS correlates with sex and age [32]. In our study, when unmatched for sex and age, there were a higher portion of males in the subclinical systolic dysfunction group (GLS < 18%), and these individuals had higher muscle and fat content, compared to the control group (GLS ≥ 18%). However, after adjusting for differences in sex, the difference in muscle content became insignificant. Our study therefore emphasizes the role of fat in early cardiac function changes in a relatively young population, regardless of sex.
The risk presented by abdominal obesity in CVD appears to be driven by metabolic abnormalities. We found that in addition to waist circumference and adiposity, indicators of metabolic disorders, such as triglycerides and uric acid, were also elevated in the subclinical impaired systolic function group. Previous studies identified risk factors for heart failure in the preclinical phase, especially in patients with diabetes mellitus, with HbA1c, triglycerides, uric acid, heart rate, BMI, and epicardial adipose tissue size being some of the major factors associated with this disorder. Obesity-induced metabolic abnormalities, such as insulin resistance, may be a common cause of altered cardiac function [33,34,35,36].
The pathophysiological mechanism of cardiac dysfunction induced by abdominal fat remains unclear. Excessive abdominal fat may be a sign of increased visceral fat. Abdominal fat can act as an endocrine organ, causing inflammation and elevation of adipokines, as well as inducing ectopic deposition of fat [37]. It has been demonstrated that systemic inflammation can cause endothelial dysfunction, which results in abnormal longitudinal strain, since longitudinal strain is a reflection of the function of the longitudinal subendocardial fibers within the heart.[38]. Adipokines may also have direct effects on the myocardium [39]. Although subcutaneous fat seems to be more beneficial than visceral fat, several studies indicate that abdominal subcutaneous adipose tissue plays a significant role in the etiology of insulin resistance, acting as an endocrine organ and causing inflammation and the elevation of adipokines [40,41,42]. However, more studies are needed to confirm this.
In the context of clinical management of people with T2DM, our findings support the concept that regional fat distribution would appear to be the more important target. As regional fat distribution varies widely, even mild weight loss can have remarkably positive effects. Lifestyle interventions for individuals with excess abdominal and visceral fat have resulted in a rapid reduction in abdominal and visceral fat, albeit with only a moderate weight loss [43]. Despite glucose-lowering medications being the primary pharmacological treatment for diabetes mellitus, data on the effects of these medications on abdominal fat are scarce. In a sub-study of the SUSTAIN 8 study, a reduction in visceral fat mass was observed in both the treatment group receiving the glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide and the treatment group receiving the sodium-glucose transport protein 2 (SGLT2) inhibitor canagliflozin [44]. SGLT2 inhibitors have shown significant clinical benefits in reducing the risk of CVD. A systematic review of four randomized controlled trials (EMPA-REG, OUTCOME, CANVAS, and DECLARE-TIMI 58) involving a total of 34,322 patients was published in 2019, with the aim to evaluate the efficacy of SGLT2 inhibitors in reducing the risk of death due to 3P-MACE (3-point major adverse cardiac event: myocardial infarction, stroke, or CVD) and hospital admissions due to heart failure and renal disease progression [45]. The authors reported that the reduction in hospital admissions due to heart failure and renal disease progression was evident regardless of the presence of atherosclerotic CVD or heart failure at baseline. Another systematic review showed that body weight significantly decreased in patients with T2DM who received different dosages of SGLT2 inhibitors compared with patients who received a placebo. Moreover, in patients treated with dapagliflozin, there was a statistically significant dosage-dependent trend in body weight reduction [46]. The implications of these findings are particularly relevant for Asians in whom increased abdominal obesity is a known factor in the development of insulin resistance and subsequent risk of cardiovascular dysfunction.
Our study results verify the correlation between abdominal fat and early impaired systolic function in the preclinical phase, possibly suggesting that abdominal fat is a risk factor to be targeted for preventing heart failure in the asymptomatic phase. However, it is important to note that our study has several potential limitations. Firstly, it was a cross-sectional investigation conducted at a single medical center, which may have introduced selection bias and limited the generalizability of the results to other populations or regions. Additionally, the study did not control for potential confounding lifestyle factors, such as dietary patterns or physical activity levels, which may have impacted fat mass. Secondly, the limited sample size prevented sub-group analyses based on insulin or oral medication types, and only examined the association between fat mass and global longitudinal strain.
Future research may include prospective studies with larger sample sizes, longitudinal designs to track changes over time, and more detailed assessments of lifestyle factors and medication regimens. It is also important to note that our findings add to the growing body of evidence highlighting the impact of adiposity on cardiac function and the need for interventions targeting abdominal fat in individuals at risk of CVD.
Conclusions
Fat mass, especially android fat mass, is closely related to subclinical left ventricular systolic dysfunction. This finding indicates a potential risk factor in the treatment of patients with T2DM without established cardiac disease.
References
Raghavan S, Vassy JL, Ho Y, et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8: e011295.
Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140:e294-324.
Peterson LR, McKenzie CR, Schaffer JE. Diabetic cardiovascular disease: getting to the heart of the matter. J Cardiovasc Transl Res. 2012;5:436–45.
de Couto G, Ouzounian M, Liu PP. Early detection of myocardial dysfunction and heart failure. Nat Rev Cardiol. 2010;7:334–44.
Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease. Circ Heart Fail. 2015;8:448–54.
Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non-ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016;18:1331–9.
Klaeboe LG, Edvardsen T. Echocardiographic assessment of left ventricular systolic function. J Echocardiogr. 2019;17:10–6.
Kato T, Harada T, Kagami K, Obokata M. The roles of global longitudinal strain imaging in contemporary clinical cardiology. J Med Ultrason. 2022;49:175–85.
Stanton AM, Vaduganathan M, Chang L-S, Turchin A, Januzzi JL, Aroda VR. Asymptomatic diabetic cardiomyopathy: an underrecognized entity in type 2 diabetes. Curr Diab Rep. 2021;21:41.
Tromp J, Tay WT, Ouwerkerk W, et al. Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN-HF registry. PLOS Med. 2018;15:e1002541.
Chandramouli C, Tay WT, Bamadhaj NS, et al. Association of obesity with heart failure outcomes in 11 Asian regions: a cohort study. PLOS Med. 2019;16:e1002916.
Schneider HJ, Friedrich N, Klotsche J, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95:1777–85.
Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41:221–6.
Hatani Y, Tanaka H, Mochizuki Y, et al. Association of body fat mass with left ventricular longitudinal myocardial systolic function in type 2 diabetes mellitus. J Cardiol Neth. 2020;75:189–95.
Messina C, Albano D, Gitto S, et al. Body composition with dual energy X-ray absorptiometry: from basics to new tools. Quant Imaging Med Surg. 2020;10:1687–98.
Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
Holland DJ, Marwick TH, Haluska BA, et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart. 2015;101:1061–6.
Ernande L, Bergerot C, Rietzschel ER, et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J Am Soc Echocardiogr. 2011;24:1268-1275.e1.
Iskander J, Kelada P, Rashad L, Massoud D, Afdal P, Abdelmassih AF. Advanced echocardiography techniques: the future stethoscope of systemic diseases. Curr Probl Cardiol. 2022;47: 100847.
Huang SJ, Orde S. From speckle tracking echocardiography to torsion. Curr Opin Crit Care. 2013;19:250–7.
Dong Y, Chen Z, Gong Y, et al. The risks of cardiovascular disease following weight change in adults with diabetes: a cohort study and meta-analysis (jc.2022–01089). J Clin Endocrinol Metab. 2022;107:dgac485.
Kishi S, Armstrong AC, Gidding SS, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling. JACC Heart Fail. 2014;2:500–8.
Suto M, Tanaka H, Mochizuki Y, et al. Impact of overweight on left ventricular function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2017;16:145.
Thomas E, Gupta PP, Fonarow GC, Horwich TB. Bioelectrical impedance analysis of body composition and survival in patients with heart failure. Clin Cardiol. 2019;42:129–35.
Oreopoulos A, Ezekowitz JA, McAlister FA, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. 2010;85:609–17.
Selvaraj S, Martinez EE, Aguilar FG, et al. Association of central adiposity with adverse cardiac mechanics. Circ Cardiovasc Imaging. 2016;9:e004396.
Szulc P, Duboeuf F, Chapurlat R. Age-related changes in fat mass and distribution in men—the cross-sectional STRAMBO study. J Clin Densitom. 2017;20:472–9.
Andrén B, Lind L, Larsson K, Hedenstierna G, Ljunghall S, Lithell H. The influence of body composition on left ventricular mass and other echocardiographic and Doppler measurements in 70-year-old males. Clin Physiol. 1995;15:425–33.
Garg PK, Biggs ML, Kizer JR, Shah SJ, Djousse L, Mukamal KJ. Associations of body size and composition with subclinical cardiac dysfunction in older individuals: the cardiovascular health study. Int J Obes. 2021;45:2539–45.
Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–4.
Skaarup KG, Lassen MCH, Johansen ND, et al. Age- and sex-based normal values of layer-specific longitudinal and circumferential strain by speckle tracking echocardiography: the Copenhagen City Heart Study. Eur Heart J - Cardiovasc Imaging. 2022;23:629–40.
Silverii GA, Toncelli L, Casatori L, et al. Assessment of left ventricular global longitudinal strain in patients with type 2 diabetes: Relationship with microvascular damage and glycemic control. Nutr Metab Cardiovasc Dis NMCD Neth. 2022;32:994–1000.
Liu J-H, Wu M-Z, Li S-M, et al. Association of serum uric acid with biventricular myocardial dysfunction in patients with type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis NMCD Neth. 2021;31:2912–20.
Yamauchi Y, Tanaka H, Yokota S, et al. Effect of heart rate on left ventricular longitudinal myocardial function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20:87.
Antakly-Hanon Y, Ben Hamou A, Garçon P, et al. Asymptomatic left ventricular dysfunction in patients with type 2 diabetes free of cardiovascular disease and its relationship with clinical characteristics: the <scp>DIACAR</scp> cohort study. Diabetes Obes Metab. 2020;23:434–43.
Neeland IJ, Ross R, Després J-P, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7:715–25.
Badimon L, Romero JC, Cubedo J, Borrell-Pagès M. Circulating biomarkers. Thromb Res. 2012;130:S12–5.
Abel ED, Sweeney G. Modulation of the cardiovascular system by leptin. Biochimie. 2012;94:2097–103.
Tordjman J, Divoux A, Prifti E, et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol. 2012;56:1152–8.
de Mutsert R, Gast K, Widya R,et al. Associations of abdominal subcutaneous and visceral fat with insulin resistance and secretion differ between men and women: the Netherlands epidemiology of obesity study. Metab Syndr Relat Disord. 2018;16:54–63.
Goodpaster BH, Leland Thaete F, Simoneau J-A, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85.
Ross R. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med. 2000;133:92.
McCrimmon RJ, Catarig A-M, Frias JP, et al. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63:473–85.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9. https://doi.org/10.1016/S0140-6736(18)32590-X. (Erratum in: Lancet. 2019;393(10166):30).
Cai X, Yang W, Gao X, et al. The association between the dosage of SGLT2 inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity. 2017;26:70–80.
Acknowledgements
Funding
The study and the Rapid Service Fee were supported by grants from the National Natural Science Foundation of China (Grant no.: 82200924), Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (Grant no.: YKK20062), Funding for Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (Grant no.: 2022-YXZX-NFM-01), and the Diabetes Mellitus Research Fund program_II_04 from Shanghai Medical and Health development foundation
Author Contributions
Dalong Zhu, Ran Meng, Ning Zhang, and Fan Yang conceived and designed the study. Jie Liu, Qichao Sun, Ning Zhang, and Jing Yao contributed to data collection and supported the recruitment of patients. Jie Liu analyzed the data and wrote the manuscript. Dalong Zhu, Ran Meng, Fan Yang, and Tianwei Gu revised the manuscript. All authors read and approved the final manuscript. Each person listed as an author or coauthor meets all four criteria formulated by the International Committee of Medical Journal Editors.
Disclosures
Jie Liu, Fan Yang, Qichao Sun, Tianwei Gu, Jing Yao, Ning Zhang, Ran Meng, Dalong Zhu declare that they have no competing interests.
Compliance with Ethics Guidelines
The study was performed in accordance with the Helsinki Declaration of 1964 and its subsequent amendments. Ethical approval was provided by the Nanjing Drum Tower Hospital Ethics Committee (No. 2021-573-01). All participants provided written informed consent to participate.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding authors
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, J., Yang, F., Sun, Q. et al. Fat Mass is Associated with Subclinical Left Ventricular Systolic Dysfunction in Patients with Type 2 Diabetes Mellitus Without Established Cardiovascular Diseases. Diabetes Ther 14, 1037–1055 (2023). https://doi.org/10.1007/s13300-023-01411-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01411-7