Abstract
Glucocorticoids, also known as steroids, are a class of anti-inflammatory drugs utilised widely in clinical practice for a variety of conditions. They are associated with a range of side effects including abnormalities of glucose metabolism. Multiple guidelines have been published to illustrate best management of glucocorticoid-induced hyperglycaemia and diabetes in a variety of settings. This article discusses current best clinical practice including diagnosis, investigations and ongoing management of glucocorticoid-induced dysglycaemia in both in- and outpatient settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glucocorticoid-induced dysglycaemia is often underdiagnosed. |
Complications are costly to the health system and the individual. |
Early diagnosis and management are key to preventing significant morbidity and mortality. |
Knowledge gaps include guidance on optimal diagnosis and monitoring in community and prognosis in the context of Covid-19. |
Introduction
More than 10% of all inpatients in the UK are treated with glucocorticoids [1, 2]. Dysglycaemia associated with glucocorticoid use is under-recognised and -monitored [3]. Hyperglycaemia can lead to a range of complications and is associated with increased mortality and prolonged hospital stays. Despite guidelines recommending glucose monitoring, a UK-based study found less than one third of patients received any estimation of glucose level before initiation of glucocorticoids [3]. Increasing use of glucocorticoids during the Covid-19 pandemic has highlighted the need for recognition and management of glucocorticoid-related dysglycaemia.
This practical guide is a study of existing literature and guidelines with no new study of human or animal subjects; therefore, ethical clearance was not sought. This article is limited to exogenous use of glucocorticoids and does not discuss hyperglycaemia related to endogenous glucocorticoids.
This is a study of existing literature and guidelines with no new study of human or animal subjects; therefore, ethical clearance was not sought.
Definitions of Glucocorticoid-induced Hyperglycaemia and Diabetes
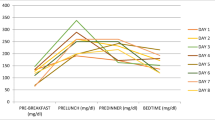
Worsening glycaemic control in the context of pre-existing diabetes mellitus is termed glucocorticoid-induced hyperglycaemia whereas hyperglycaemia in the absence of pre-existing diabetes is termed glucocorticoid-induced diabetes. Hyperglycaemia is defined as blood glucose (BG) levels > 11.1 mmol/l on at least teo occasions after commencing glucocorticoid therapy (Fig. 1).
Pathophysiology
Glucocorticoids contribute to hyperglycaemia via disruption of various physiological metabolic mechanisms by both increasing insulin resistance and decreasing insulin secretion (Fig. 2). Insulin production is reduced because of decreased expression of glucose transporter type 2 (GLUT2) and glucokinase receptors on pancreatic beta cells.
Reduced insulin sensitivity results from the effects of glucocorticoids on the liver, skeletal muscle and fat cells. The liver plays an important role in maintaining glycaemic control through glucose production (gluconeogenesis) and glycogen store breakdown (glycogenolysis). Glucocorticoids induce enzymes that promote gluconeogenesis as well as increase lipolysis and proteolysis. Skeletal muscle represents the largest reserve of glycogen in the body, storing 80% of postprandial glucose. Glucocorticoids interfere with signalling cascades via glucose transporter type 4 (GLUT4), similarly to the glucose transporter, which is responsible for clearance of glucose post-prandially in response to insulin. This interference results in reduced glucose uptake in muscle cells and reduced glycogen synthesis. Glucocorticoids also promote lipolysis leading to an increase in serum-free fatty acids and triglycerides as well as causing deposition of fat in organs and reducing peripheral reserves [7].
Risk Factors
The risk of developing diabetes with use of glucocorticoids is almost doubled with estimates of relative risk ranging from 1.36 to 2.31 [8, 9]. Meta-analysis data have demonstrated that 32.3% of individuals treated with glucocorticoids develop hyperglycaemia and 18.6% develop glucocorticoid-induced diabetes [2]. Both person-specific and pharmacological factors can confer a higher risk of glucocorticoid-induced dysglycaemia (Box 1). Identifying individuals at risk could aid targeted diagnostic testing and monitoring.
Multiple person-specific factors relate to increased risk of dysglycaemia in the context of glucocorticoid use. These factors include older age, raised body mass index (BMI), raised HbA1c, personal history of gestational diabetes and family history of diabetes. Additionally, individuals at risk of diabetes or those with impaired glucose metabolism are also at increased risk of glucocorticoid-induced dysglycaemia.
Pharmacological factors should be considered in identifying those at risk. Complications of glucocorticoids appear to be dose dependent with higher risks of complications associated with potency, duration and dosage. Evidence suggests that any formulation of glucocorticoid can affect glycaemic control as an association of hyperglycaemia with oral, inhaled, topical, intra-articular and intralesional routes of administration [6]. Supraphysiological doses equating to ≥ 5 mg of prednisolone are related to glucocorticoid side effects; however, Joint British Diabetes Societies (JBDS) guidelines advise caution because some individuals may develop side effects at lower doses. Prolonged courses confer greater risk and estimates suggest that around 22% of glucocorticoid use lasts > 6 months and 4.3% > 5 years [10].
Clinical Relevance
Glucocorticoid-induced hyperglycaemia and diabetes are associated with increased morbidity and mortality and increased healthcare costs. Hyperglycaemia can result in osmotic symptoms and can lead to acute complications such as diabetic ketoacidosis (DKA) and hyperosmolar hyperglycaemic state (HHS). Hyperglycaemia is associated with poorer outcomes including longer or more frequent hospitalisations, infection risk and increased burden of morbidity and mortality [5]. Long-term microvascular and macrovascular risks may be exacerbated in patients who receive glucocorticoid treatment and the co-existence of inflammatory diseases with glucocorticoid-induced hyperglycaemia confers increased cardiovascular risk [7].
Glucocorticoid-induced hyperglycaemia and diabetes are underdiagnosed and under-monitored. A study of adult patients in the UK prescribed systemic glucocorticoids for > 3 months observed variations in glucose monitoring across the country. They found that less than one third of patients received glucose monitoring prior to commencing glucocorticoids and < 60% of patients prescribed glucocorticoids for over a year received some glucose monitoring after exposure [3]. This may in part be due to a lack of consensus guidance on community- and outpatient-based recommendations on diagnosis and monitoring.
The Covid-19 pandemic has further highlighted the clinical relevance of glucocorticoid-induced hyperglycaemia. The pandemic led to an increase in hospital admissions with viral-induced acute illness as well as an increase in the use of glucocorticoids. Covid-19 has been shown to lead to higher rates of hyperglycaemia and has been associated with higher rates of new-onset diabetes, DKA and HHS [12]. The mechanisms of these findings are still not fully known but may relate at least in part to acute illness and glucocorticoid use. Hospitalised patients with Covid-19 have an increased risk of all-cause mortality in the presence of newly diagnosed or pre-existing diabetes [12].
Management
Blood Glucose Monitoring and Targets
Oral glucose tolerance tests and HbA1c measurement are not routinely suggested for use in the screening/diagnosis of glucocorticoid-induced diabetes in the acute setting unless otherwise indicated. HbA1c may be inaccurate or misleading in the acute setting of new-onset hyperglycaemia where glycaemic control had been within normal limits prior to administration of glucocorticoids. HbA1c should be checked prior to commencement of glucocorticoid therapy in those deemed to be at high risk of hyperglycaemia to exclude pre-existing diabetes in these groups.
In hospital, those with known diabetes should have blood glucose checked four times daily. In individuals not known to have diabetes, glucose should be estimated once daily upon commencement of glucocorticoid therapy. In general, a morning dose of glucocorticoid will likely result in an early afternoon transient increase in serum glucose. Blood glucose monitoring at this time therefore offers the greatest diagnostic sensitivity. However, evidence suggests that 2-h post-prandial glucose measurements at any time of day can reliably be used to detect glucocorticoid-induced hyperglycaemia with any regimen [5]. If glucose exceeds 11.1 mmol/l, glucose monitoring should be increased to four times a day.
In general, a target blood glucose level between 6 and 10 mmol/l is recommended in the inpatient population. In groups with high risk of hypoglycaemia or vulnerable to harm from hypoglycaemia, strict glycaemic targets may not be indicated and levels between 6 and 15 mmol/l may be acceptable. Vulnerable patient groups include people with dementia, patients with delirium or frailty, those with recent brain injury, those with risk of falling, renal impairment and dialysis, patients with variable dietary intake and those at end-of-life care. In end-of-life care, management should be focussed on symptomatology.
The major consensus guidelines on diagnosis and management of glucocorticoid-induced hyperglycaemia and diabetes are largely targeted towards inpatients. However, a large proportion of glucocorticoids is commenced in outpatient and community settings. JBDS guidelines recommend increased monitoring in those with known diabetes and commencing monitoring in high-risk groups. A pragmatic approach in the primary care setting is to ensure that all high-risk individuals and those with pre-existing diabetes have access to glucose monitoring at home with twice weekly monitoring as minimum. Monitoring should be more rigorous if levels exceed 11.1 mmol/l and all patients should receive education about hyperglycaemic symptoms which should prompt testing. Undiagnosed diabetes should be excluded in high-risk groups by a definitive test for diabetes prior to commencing glucocorticoids. These measures would ideally reduce acute presentations of hyperglycaemia in secondary care and allow earlier intervention of hyperglycaemia [13]. Guidelines specific for cancer patients recommend monitoring glucose levels at every outpatient appointment if considering systemic anti-cancer or glucocorticoid therapy [14, 15].
Pharmacological Management
T1DM
It is recommended to titrate regular insulin by 10–20% as required to achieve glucose targets (Fig. 2). In basal-bolus regimens the evening basal insulin can be transferred to the morning and basal doses increased by 10–20% daily until targets are met [4]. If hyperglycaemia remains persistent despite insulin titration, specialist input may be required to consider adjustment of insulin therapy.
T2DM
Recommendations suggest commencing once daily morning gliclazide 40 mg and titrating as required until glycaemic control is achieved. If at maximal dose of morning gliclazide it is recommended to commence an additional evening dose or consider commencing insulin therapy. Once daily intermediate-acting insulin in the morning is recommended initially but twice daily or basal bolus regimens can be considered to achieve glucose targets. In those already on insulin therapy, doses can be titrated up by 10–20% to achieve targets and evening basal doses can be transferred to the morning (Figs. 3, 4).
Glucocorticoid-induced Hyperglycaemia
Gliclazide 40 mg once daily in the morning is recommended and can be titrated up to a maximum dose of 240 mg once daily in the morning as per BG targets. If glycaemic control is not achieved, evening dose of gliclazide can be added and intermediate-acting insulin considered.
Diabetic Emergencies
In cases presenting with significant hyperglycaemia, insulin may be required initially and variable-rate insulin infusion may be indicated [16]. Those presenting with DKA or HHS should be managed as per local guidelines with input from the specialist team. In complex cases, such as those on parenteral nutrition, early input from the diabetic team may be indicated to tailor pharmacotherapy [16].
Cessation of Glucocorticoid Therapy
When glucocorticoid therapy is stopped, consideration of ongoing glucose monitoring and review of ongoing glucose lowering therapy should be made. Sulphonylurea and insulin doses should be reduced in tandem with reductions in glucocorticoid dose to avoid hypoglycaemia. Glucose monitoring should be continued until euglycaemia is achieved. For hospital patients discharged with glucocorticoids, it is advised to continue a minimum of once daily glucose monitoring upon discharge. In those who have discontinued glucocorticoids but hyperglycaemia persists, glucose monitoring should be continued and after 3 months formal testing for diabetes should be undertaken. Newly diagnosed patients may require devices for glucose monitoring upon discharge and be provided with education on glucose monitoring including safety netting advice if persistent hyperglycaemia or hypoglycaemia exists [16].
Prognosis
After stopping glucocorticoid therapy up to a third of people with glucocorticoid-induced diabetes may go on to develop persistent diabetes [4]. There is a degree of diagnostic uncertainty in cases of steroid-induced diabetes where baseline glucose levels and/or HbA1c have not been checked prior to exposure to glucocorticoids. There are some data to suggest that glucocorticoids unmask pre-existing glucose metabolism disorders thus leading to persistent diabetes [17, 18]. Chronic exposure to glucocorticoids can lead to metabolic and cardiovascular adverse events with the risk of diabetes and cardiovascular events increased two- to three-fold [3]. Over 50% of individuals taking chronic glucocorticoid treatment develop features of metabolic syndrome further contributing to cardiovascular risk [19]. The prognosis of glucocorticoid complications in the context of Covid-19 is not yet known and further prospective studies into this are required.
Conclusion
Despite the wide clinical usage of glucocorticoid therapy and well-documented evidence of glucocorticoid-induced hyperglycaemia and diabetes, these complications still go under-diagnosed and -monitored. Consensus guidelines have a strong focus on hospital inpatient management despite widespread community use; however, clinical evidence provides pragmatic solutions for monitoring and usage in the community. Lack of early diagnosis and appropriate management is costly to both the patient in the short and long term in addition to the health system as a whole.
References
Narwani V, Swafe L, Stavraka C, Dhatariya K. How frequently are bedside glucose levels measured in hospital inpatients on glucocorticoid treatment? Clin Med. 2014;14(3):327–8.
Liu XX, Zhu XM, Miao Q, Ye HY, Zhang ZY, Li YM. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65(4):324–32.
Fardet L, Petersen I, Nazareth I. Monitoring of patients on long-term glucocorticoid therapy: a population-based cohort study. Medicine. 2015;94(15): e647.
Roberts A, James J, Dhatariya K, Joint British Diabetes Societies (JBDS) for Inpatient Care. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet Med. 2018;35(8):1011–7.
Bonaventura A, Montecucco F. Steroid-induced hyperglycemia: an underdiagnosed problem or clinical inertia? A narrative review. Diabetes Res Clin Pract. 2018;139:203–20.
Fathallah N, Slim R, Larif S, Hmouda H, Ben SC. Drug-induced hyperglycaemia and diabetes. Drug Saf. 2015;38(12):1153–68.
Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes. 2015;6(8):1073–81.
Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care. 2006;29(12):2728–9.
Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the elderly. J Gen Intern Med. 2002;17(9):717–20.
Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology. 2011;50(11):1982–90.
Caplan F, Rosenbach W. Prevention and management of glucocorticoid-induced side effects: A comprehensive review: Ocular, cardiovascular, muscular, and psychiatric side effects J Am Water Works Assoc [Internet]. Available from: https://www.sciencedirect.com/science/article/pii/S0190962216300433?casa_token=8kYB5W6Bs7gAAAAA:ud-DntOba0qFlds_91igeap7m6eou1sMnmhCvkbQK1gHPrWrJJwrKszKo_ukcvqtmhJ-2zYv6WA.
Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care. 2021;44(12):2645–55.
Mills E, Devendra S. Steroid-induced hyperglycaemia in primary care. Lond J Prim Care (Abingdon). 2015;7(5):103–6.
Joharatnam-Hogan N, Chambers P, Dhatariya K, Board R, Joint British Diabetes Society for Inpatient Care (JBDS), UK Chemotherapy Board (UKCB). A guideline for the outpatient management of glycaemic control in people with cancer. Diabet Med. 2022;39(1):e14636.
Sinaga G, de Koeijer E. Management of dexamethasone-induced hyperglycemia in patients undergoing chemotherapy in an outpatient setting: a best practice implementation project. JBI Database Syst Rev Implement Rep. 2018;16(4):1068–78.
Shah P, Kalra S, Yadav Y, Deka N, Lathia T, Jacob JJ, et al. Management of Glucocorticoid-Induced Hyperglycemia. Diabetes Metab Syndr Obes. 2022;23(15):1577–88.
Simmons LR, Molyneaux L, Yue DK, Chua EL. Steroid-induced diabetes: is it just unmasking of type 2 diabetes? ISRN Endocrinol. 2012;5(2012): 910905.
Wu J, Mackie SL, Pujades-Rodriguez M. Glucocorticoid Dose-Dependent Risk of Type 2 Diabetes in Six Immune-Mediated Inflammatory Diseases: A Population-Based Cohort Analysis [Internet]. SSRN Electron J. https://doi.org/10.2139/ssrn.3501035.
Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing’s syndrome: cohort study. BMJ. 2012;30(345): e4928.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The rapid service fee was kindly waivered by Diabetes Therapy editorial team.
Author Contributions
All authors contributed to the writing and design of this manuscript. The first draft of the manuscript was written by Hannah Barker and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Hannah L. Barker, Deborah Morrison, Andrea Llano, Christopher A.R. Sainsbury, Gregory C. Jones have nothing to disclose.
Compliance with Ethics Guidelines
This is a study of existing literature and guidelines with no new study of human or animal subjects therefore ethical clearance has not been sought.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed within this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Barker, H.L., Morrison, D., Llano, A. et al. Practical Guide to Glucocorticoid Induced Hyperglycaemia and Diabetes. Diabetes Ther 14, 937–945 (2023). https://doi.org/10.1007/s13300-023-01393-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01393-6