Abstract
Introduction
Liraglutide has demonstrated a significant reduction in the primary major composite cardiovascular (CV) outcome (CV death, non-fatal myocardial infarction, non-fatal stroke). This study aimed to determine the cost–utility of adding liraglutide to the standard of care (SoC) for treating type 2 diabetes (T2D) in Thailand for three cohorts: people with atherosclerotic cardiovascular disease (ASCVD), with no ASCVD, and all people with T2D.
Methods
A Markov model was developed to capture the long-term costs and outcomes under the perspective of the healthcare system. Costs were based on local data, the transitional probabilities were derived from the LEADER trial, and utilities were derived from published studies. Future costs and outcomes were discounted at 3% annually. A series of sensitivity analyses were performed.
Results
Compared to SoC, adding liraglutide incurred higher costs and gained more quality-adjusted life-years (QALYs), yielding incremental cost-effectiveness ratios (ICERs) of above 1 million Thai baht (THB) for the three cohorts. The most influential parameter was the discount rate. When the annual cost of liraglutide reduced from 87,874 to 30,340 THB, 30,116 THB, and 31,617 THB for all people with T2D, people with ASCVD, and people without ASCVD, respectively, the ICER fell below the local threshold of 160,000 THB/QALY. Compared to the SoC treatment, the liraglutide group acquired more clinical benefit in terms of fewer CVD. Sensitivity analyses revealed that with an increase in the level of willingness-to-pay (WTP) threshold, adding liraglutide had an increased chance of being a cost-effective strategy.
Conclusion
Compared to the SoC treatment, adding liraglutide at the current cost is not cost-effective at the local WTP. People with T2D with ASCVD would have the most potential gain from adding liraglutide treatment compared to other populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The prevalence of type 2 diabetes (T2D) is increasing, and the associated complications place a substantial cost burden on the healthcare system in Thailand. |
Liraglutide has shown significant decrease in the risk of cardiovascular (CV) outcomes which may improve healthcare spending on long-term complications. |
However, there is paucity of data on local economic evidence based on the cardiorenal protection benefits from the LEADER study. |
What was learned from this study? |
Adding-on liraglutide to standard of care (SoC) showed a higher incremental cost-effectiveness ratio (ICER) which was above the local willingness-to-pay (WTP) threshold versus SoC alone but shown to save 77 CV deaths which yielded an ICER above the acceptable ceiling in Thailand. |
Liraglutide would be considered cost-effective at an annual treatment cost of around 30,000 THB/year in Thailand. |
People with type 2 diabetes (T2D) with atherosclerotic cardiovascular disease (ASCVD) would have the most potential gain from adding liraglutide treatment compared to other populations characterized in this study. |
Introduction
Type 2 diabetes mellitus (T2D) is a growing public health issue globally. In 2021, T2D affected approximately 536.6 million people worldwide, and is estimated to increase to 783.2 million by 2045 [1]. T2D, especially with poor glycemic control, is strongly associated with a risk of micro- and macrovascular complications and mortality [2]. These comorbidities place a substantial cost burden on the healthcare system. In Thailand, 6.1 million adults are affected by diabetes [1]. According to the 5th National Health Examination Survey, almost 8.9% and 10.8% of Thai men and women, respectively, were affected by T2D among which less than half (45.9% and 36.4%, respectively) received any T2D treatment [3]. Additionally, approximately 44% of Thai people with T2D reported a history of microvascular complications, and 6% reported a history of cardiovascular disease (CVD) [4]. About half of the direct medical cost was allocated to hospital care, whereas the cost of medicine accounted for only 14%. Moreover, the cost of treating people with diabetes increased exponentially when those people developed complications [4, 5]. Clinical guidelines recognize the importance of management of people with T2D beyond glycemic control by counting risks and complications [6]. Glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose co-transporter 2 (SGLT2) inhibitors are part of the treatment plans for people with T2D with increased cardiovascular (CV) risks [6]. The current guideline-based practice recommends SGLT2 inhibitors or GLP-1 receptor agonists for adults with T2D and CVD or renal disease. For those with established CVD and renal disease, the guideline recommends SGLT2 inhibitors and GLP-1 receptor agonists as an alternative [7]. Liraglutide, an analogue of human GLP-1, has been approved for the treatment of T2D [8]. Liraglutide has demonstrated good glucose-lowering effects and is also associated with reductions in body weight and blood pressure [8]. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) is a large CV outcome trial of liraglutide compared to placebo as an add-on treatment to the standard treatment, including 9340 people with T2D and established CVD or multiple risk factors for CVD [9]. Over the follow-up period of 3.8 years, liraglutide demonstrated a significant reduction in the primary composite CV outcome by 13.0% (hazard ratio [HR], 0.87; 95% confidence interval [CI], 0.78–0.97; p = 0.01 for superiority). Death from CV causes occurred in fewer people in the liraglutide group (HR 0.78; 95% CI 0.66–0.93; p = 0.007) versus placebo. The data also suggested beneficial effects on the composite outcome of renal or retinal microvascular events, with results being driven by lower rates of nephropathy with liraglutide compared to placebo (HR 0.78; 95% CI 0.67–0.92; p = 0.003) [10]. These clinical benefits of liraglutide could consequently improve healthcare spending on long-term treatment for cardiorenal complications, which generally are associated with high cost. However, there is no local economic evidence based on the cardiorenal protection benefits from the LEADER study. Hence, this analysis of the LEADER trial assessed the cost–utility of adding liraglutide to the standard treatment for treating people with T2D in a Thai context.
Methods
Cohort Population
The cohort population in this study consisted of people with T2D. The study assessed the cost-effectiveness of liraglutide in three cohorts: (1) all people with T2D; (2) people with T2D with atherosclerotic cardiovascular disease (ASCVD); and (3) people with T2D but no documented ASCVD (no ASCVD).
Intervention and Comparator
The intervention group consisted of people with T2D who received 1.8 mg of liraglutide subcutaneously once-daily as an add-on to standard of care (SoC). The comparator group received SoC for T2D.
Model Structure
A Markov model, as shown in Fig. 1, was developed in Microsoft Excel and based on the CV and renal outcomes of the LEADER trial [9, 10]. The model comprised eight health status: T2D with CV risk, non-fatal myocardial infarction (MI), post-MI, non-fatal stroke, post-stroke, pre-renal replacement therapy (pre-RRT), RRT, and death. The assumption in this model was that MI, stroke, and nephropathy were mutually exclusive events. Deaths included fatal MI, fatal stroke, CV death, non-CV death, and renal death. To capture the long-term costs and outcomes, a lifetime horizon with a yearly cycle length was used for the analyses.
Each cohort was started at the T2D with CV risk health state. People then moved to non-fatal MI, non-fatal stroke, pre-RRT, or death according to the transitional probability of such a health state. People who experienced either non-fatal MI or non-fatal stroke could move to post-MI or post-stroke, respectively, or experience death from CV causes in the next cycle. People with either post-MI or post-stroke could remain in the same health state or get worse and attain death. Another pathway was nephropathy. The cohort could move from the starting health state to the pre-RRT health state or attain death from renal disease. People were then moved to the RRT health state or could remain in the pre-RRT health state or death state according to the transitional probabilities. People in the RRT health state could stay in the RRT health state or get worse and die. Finally, all people could enter the absorbing health state, which is the death state.
Input Parameters
Transitional Probability Inputs
Myocardial Infarction
Risk of non-fatal MI was obtained from the LEADER trial [9, 11], which reported 6.02% vs. 6.79% in the liraglutide and SoC groups for all people, 6.88% vs. 7.95% for people with ASCVD, and 3.72% vs. 3.77% for people without ASCVD. The number of fatal MI was fewer than non-fatal MI in all three cohorts. People who received add-on liraglutide had fewer fatal MI than those who received SoC alone in all three cohorts. The reported percentages of fatal MI were 0.36% vs. 0.60% in the liraglutide and SoC groups for all people, 0.38% vs. 0.56% for people with ASCVD, and 0.32% vs. 0.69% for people without ASCVD [9, 11]. All these risks of non-fatal MI and fatal MI were converted into an annual rate, then converted back to an annual probability. The details are shown in Table 1.
Stroke
The data on non-fatal stroke and fatal stroke were also obtained from the LEADER trial [9, 11]. The number of non-fatal strokes was higher compared to fatal strokes for all three cohorts. People who received liraglutide treatment had lower percentages of non-fatal stroke versus those who received SoC treatment (3.67% vs. 4.33% for people with ASCVD, 3.41% vs. 3.79% for all people). However, people from the no ASCVD cohort had higher percentages of non-fatal stroke in the liraglutide group than the SoC group (2.69% vs. 2.38%). There were fewer people with fatal stroke in the liraglutide group than in the SoC group for all three cohorts. Risks of fatal stroke in the liraglutide and SoC groups were 0.34% vs. 0.54%, 0.32% vs. 0.56%, and 0.40% vs. 0.46% for all people, people with ASCVD, and people without ASCVD, respectively. All these risks of non-fatal stroke and fatal stroke were converted to an annual rate, then converted back to an annual probability. The details are shown in Table 1.
Cardiovascular Death
The mortality rate after the first non-fatal event of MI and stroke in the SoC group was 4.8 per 100 patient-year based on the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care (EXAMINE) study [12]. The mortality rate due to MI in the liraglutide group was obtained from the mortality rate of the SoC group adjusted by the HR of 0.60 (95% CI 0.33–1.10) for all people [9], 0.67 (95% CI 0.29–1.57) for people with ASCVD, and 0.45 (95% CI 0.14–1.47) for people without ASCVD [11]. All rates were finally converted into risk. The details are shown in Table 1.
RRT and Pre-RRT
The LEADER trial [10] reported one of the renal outcomes as the number of people needing RRT. People with ASCVD had a lower risk of RRT than those with no ASCVD for both the liraglutide and SoC groups. In addition, people who received liraglutide treatment had lower risk of RRT than those who received SoC in all three cohorts. The percentages of RRT in the liraglutide and SoC groups were 1.20% vs. 1.37%, 0.82% vs. 0.95%, and 2.21% vs. 2.46% for all people, people with ASCVD, and people without ASCVD, respectively. All these risks were converted to an annual rate, then to an annual probability. The details are shown in Table 1.
Since the renal outcomes reported from the LEADER trial [10] did not clearly show the rate of pre-RRT, we estimated this rate from the rate of all renal outcomes minus RRT and renal death. Then, the estimated rate of pre-RRT was converted into an annual probability. The details are shown in Table 1.
Renal Death
The mortality rates from pre-RRT and RRT of the SoC group were obtained from the United Kingdom Prospective Diabetes Study (UKPDS-64) [13], then adjusted by the HR from the LEADER trial [10]. All rates were finally converted into risk. Renal death from the initial health state and T2D with CV risk were also considered. People who received liraglutide treatment had a numerically higher risk of renal death than those who received SoC treatment. although the difference was not statistically significant and based on very low occurrence. This risk was more evident in people without ASCVD than those with ASCVD. The risk of renal death in the liraglutide and SoC groups was 0.17% vs. 0.11%, 0.12% vs. 0.09%, and 0.32% vs. 0.15% for all people, people with ASCVD, and people without ASCVD, respectively [10].
Cost Inputs
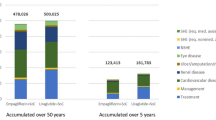
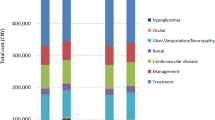
This study was conducted from a healthcare system perspective; therefore, only direct medical costs were included. The direct medical costs included costs of liraglutide and other antihyperglycemic agents for people who did not meet the recommended target for glycemic control [14, 15], cost of adverse event treatment, costs of complication treatment, and costs of CV events (including CV death).
The acquisition costs of liraglutide and other antihyperglycemic agents were obtained from the Drug and Medical Supply Information Center (DMSIC), Thailand Ministry of Public Health [16]. Liraglutide 3 mL sterile solution of 6 mg/mL costs 2407 Thai baht (THB), resulting in an annual cost of 87,874 THB. For other antihyperglycemic agents where a product was supplied by several pharmaceutical companies, the median price from the median list of all pharmaceutical companies was used, in accordance with the Thai Health Technology Assessment (HTA) Guideline [17]. The total cost was the product of unit cost of individual antihyperglycemic agent mentioned above and the resource use obtained from the LEADER trial [9]. The difference in total cost of antihyperglycemic agents from both strategies was included for data analysis. Since people discontinued liraglutide treatment at a rate of 5.23% per year, we factored it into the total cost calculation. Treatment costs of adverse events and complications were based on a published study [18] or a large university-affiliated hospital database [19] (Certificate of Approval No. EXEMPTION-6811/2019). All costs were inflated to the year 2021, using the consumer price index for the medical-care category [20].
Utility Inputs
Utility is a health-related quality-of-life measure that varies from zero, representing death, to one, representing perfect health. The lifetime was weighted by utility values to estimate the quality-adjusted life-year (QALY) associated with health states after treatments. The baseline utility was based on people without diabetes-related complications, which was 0.753 [21]. Utility decrements associated with CV and renal complications were obtained from published studies and applied in the model. Table 2 shows the utility and disutility values used in the model.
Health Outcomes
The predicted long-term costs and outcomes of interest in this study were clinical outcomes, such as number of CV deaths and renal deaths, the incremental costs, life-years gained, QALY gained, and incremental cost-effectiveness ratio (ICER).
Data Analyses
Base-Case Analysis
Of all three cohorts, the expected lifetime total cost and outcomes of add-on liraglutide to existing regimen compared to SoC in each population were calculated and discounted at an annual rate of 3% [22]. The ICER was calculated using the differences in lifetime total cost divided by the difference in outcome for both strategies. When the estimated ICER fell below the accepted threshold in Thailand (160,000 THB/QALY), the add-on liraglutide strategy was considered cost-effective.
Sensitivity Analyses
One-way sensitivity analyses under which each parameter varied were conducted to assess the robustness of the cost-effectiveness analysis. The key input parameters varied among plausible ranges. When available, the standard deviation or 95% CI was used as a range for the one-way sensitivity analysis. When no such data were available, costs, probabilities, and utilities were varied within a range of ± 20%, ± 10%, and ± 10%, respectively. All plausible ranges are summarized in Tables 1 and 2. The discount rate varied from 0 to 6%, following the recommendation of the Thai HTA Guideline [22]. The results were displayed as a tornado diagram. Willingness-to-pay (WTP) threshold analysis was conducted to estimate the cost of liraglutide that yielded ICER equal to the accepted threshold in Thailand (160,000 THB/QALY).
In addition, a probabilistic sensitivity analysis was conducted, whereby individual sets of parameter values were drawn from an appropriate statistical distribution (Tables 1 and 2), with results generated for 1000 simulation runs. The results of the analysis were displayed as a scatterplot and a cost-effectiveness acceptability curve, which graphically represented the probability of add-on liraglutide being cost-effective, compared to SoC alone, for different defined WTP thresholds.
Compliance with Ethics Guidelines
This article is been approved ethics committees and was performed in accordance with the Helsinki Declaration of 1964.
Results
Base-Case Result
All People with T2D
The lifetime total cost in the add-on liraglutide group was higher than in the SoC group (2,333,111 THB vs. 1,381,347 THB), resulting in an incremental cost of 951,764 THB. After disaggregation of the total costs, the major component cost of the total cost was the cost of liraglutide. However, liraglutide was shown to save 77 CV deaths per 1000 people with a gain of 0.91 years and 0.72 QALYs when compared with the SoC. This yielded an ICER of 1,051,626 THB/life-years or 1,326,197 THB/QALY, which is much above the acceptable ceiling ratio of 160,000 THB/QALY (Table 3).
ASCVD Population
The lifetime total cost in the add-on liraglutide group was higher than in the SoC group (2,394,153 THB vs. 1,422,284 THB), resulting in an incremental cost of 971,868 THB. The cost of liraglutide made the greatest contribution to the lifetime total cost. However, liraglutide was shown to save 86 CV deaths per 1000 people and 5 renal deaths per 1000 people with a gain of 1.00 years and 0.80 QALYs when compared with the SoC. This yielded an ICER of 968,404 THB/life-years or 1,222,381 THB/QALY, which is much above the acceptable ceiling ratio of 160,000 THB/QALY (Table 3).
No ASCVD Population
The lifetime total cost in the add-on liraglutide group was higher than in the SoC group (2,074,152 THB vs. 1,270,556 THB), resulting in an incremental cost of 803,596 THB. The cost of liraglutide accounted for the greatest component of the lifetime total cost. Compared to the SoC, people treated with liraglutide had a lower number of CV deaths per 1000 people (153 vs. 200) and a higher number of renal deaths per 1000 people (319 vs. 285). However, the add-on liraglutide group gained 0.12 more life-years or 0.16 QALYs than the SoC group. This yielded an ICER of 6,769,724 THB/life-years or 5,086,153 THB/QALY, which is much above the acceptable ceiling ratio of 160,000 THB/QALY (Table 3).
Sensitivity Analysis
The scatterplot on the cost-effectiveness plane showed that compared to the SoC treatment, add-on liraglutide treatment incurred higher costs for all three populations, with more QALY gain in two populations (all people with T2D and people with ASCVD). However, approximately 31% of a thousand iterations in people without ASCVD had fewer QALY in the add-on liraglutide group compared to the SoC group (Figs. 2, 3, and 4). At the WTP of 160,000 THB/QALY, it was unlikely that an add-on liraglutide treatment was a cost-effective strategy in the three cohort populations. The probability of add-on liraglutide being a cost-effective alternative was shown to increase with higher levels of WTP threshold (Figs. 2, 3, and 4). Of the three cohorts, an add-on liraglutide treatment in people with ASCVD yielded the highest percentage of being cost-effective at the same level of WTP (Fig. 5).
The results of one-way sensitivity analysis in all people with T2D and people with ASCVD showed that discount rate and cost of liraglutide were the top two parameters that had an impact on the estimated ICER (Figs. 6 and 7). For people without ASCVD, risk of pre-RRT was the most influential parameter, followed by the discount rate and risk of RRT (Fig. 8). When the annual cost of liraglutide reduced by 10%, the estimated ICERs declined accordingly, especially in people without ASCVD (Fig. 9). To yield the ICER of 160,000 THB/QALY, an annual cost of liraglutide should be equal to 65.5% (30,340 THB), 65.7% (30,116 THB), and 64.0% (31,617 THB) for all people with T2D, people with ASCVD, and people without ASCVD, respectively.
Tornado diagram of add-on liraglutide compared to standard of care alone in people with atherosclerotic cardiovascular disease. CV cardiovascular, ICER incremental cost-effectiveness ratio, MI myocardial infarction, QALY quality-adjusted life-year, RRT renal replacement therapy, SoC standard of care, THB Thai baht
Tornado diagram of add-on liraglutide compared to standard of care alone in people without atherosclerotic cardiovascular disease. CV cardiovascular, ICER incremental cost-effectiveness ratio, MI myocardial infarction, QALY quality-adjusted life-year, RRT renal replacement therapy, SoC standard of care, THB Thai baht
Discussion
The clinical evidence from the landmark LEADER trial [9, 10] showed that, when added to the SoC, liraglutide resulted in lower rate of the first occurrence of death from CV causes, non-fatal MI, and non-fatal stroke among people with T2D with ASCVD risk, and the development and progression of diabetic kidney disease. This evident clinical benefit was taken into consideration with overall costs of treatment for people with T2D in three cohorts (all people with T2D, people with ASCVD, and people without ASCVD). The findings of this study indicated that add-on liraglutide use had an ICER above the local WTP threshold of 160,000 THB/QALY compared to the SoC treatment alone for all three cohorts. Therefore, adding liraglutide may not be a cost-effective strategy with the current setting on total cost and WTP threshold. Among the three cohorts, adding liraglutide showed the most value for money in ASCVD populations. The findings indicated the lower rate of CV deaths, resulting in cost saving, in the add-on liraglutide treatment compared to the SoC treatment for all three cohorts. In addition, the lower rate of progression of diabetic kidney disease could be observed from the lower costs of pre-RRT and RRT treatment in the add-on liraglutide group compared to the SoC group (Table 3) for all three cohorts. However, the cost saving accrued from the lower rate of CV death and progression of diabetic kidney disease could not offset the acquisition cost of liraglutide, resulting in an abundant incremental cost and ICER. The annual cost of liraglutide should be reduced by 65.5%, 65.7%, and 64.0% for all people with T2D, people with ASCVD, and people without ASCVD, respectively, to yield the ICER within the local Thai threshold.
A cost-effectiveness study conducted in the USA [23] using data from the LEADER trial reported that liraglutide was a cost-effective strategy for people with T2D with established CVD or elevated CV risk compared to the SoC, with an ICER of US$106,749/QALY. The WTP threshold to justify the cost-effectiveness used in this study was US$150,000/QALY. Although the data from the LEADER trial was used in the two studies, the results could be different owing to several factors such as the difference in model structure, costs of treatment, and WTP threshold. When the incremental cost and incremental QALY were considered, the findings of the all people cohort in this study incurred lower incremental cost and gained more QALYs than those in the US study. With the huge difference in the level of acceptable threshold in these two studies, it is not surprising that the results were not consistent.
Several strengths and limitations were taken into consideration. First, the developed Markov model used in this study captured the long-term CV and renal outcomes of liraglutide; however, the transitional probabilities were derived from the median time of 3.84 years from the LEADER clinical trial [9] and carried forward the constant transitional probabilities. This might not truly reflect the real situation. The disease is likely to progress exponentially, especially in people with T2D and high risk of ASCVD. These people would gain benefit from early liraglutide treatment. Second, this study incorporated the cost of CV death into the analysis. Liraglutide treatment resulted in cost saving from the significantly lower CV death compared to the SoC treatment.
Conclusion
On the basis of the clinical evidence from the LEADER clinical trial with 3.84 years of median follow-up duration and the current cost of liraglutide, add-on liraglutide has an ICER of 1.3 million THB for all people with T2D, 1.2 million THB for people with T2D and ASCVD, and 5 million THB for people with T2D without ASCVD. People with T2D and ASCVD would have the most potential gain from adding liraglutide treatment compared to other populations.
Change history
31 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13300-023-01392-7
References
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183: 109119.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
Aekplakorn W, Chariyalertsak S, Kessomboon P, Assanangkornchai S, Taneepanichskul S, Putwatana P. Prevalence of diabetes and relationship with socioeconomic status in the Thai population: national health examination survey, 2004–2014. J Diabetes Res. 2018;2018:1654530.
Deerochanawong C, Ferrario A. Diabetes management in Thailand: a literature review of the burden, costs, and outcomes. Global Health. 2013;14(9):11.
Chatterjee S, Riewpaiboon A, Piyauthakit P, et al. Cost of diabetes and its complications in Thailand: a complete picture of economic burden. Health Soc Care Community. 2011;19(3):289–98.
American Diabetes Association. Standards of Medical Care in Diabetes-2022. Diabetes Care. 45:S1–264.
Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021;11(373):n1091.
Du Q, Wang YJ, Yang S, Zhao YY, Han P. Liraglutide for the treatment of type 2 diabetes mellitus: a meta-analysis of randomized placebo-controlled trials. Adv Ther. 2014;31(11):1182–95.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22.
Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48.
Verma S, Bhatt DL, Bain SC, et al. Effect of liraglutide on cardiovascular events in patients with type 2 diabetes mellitus and polyvascular disease: results of the LEADER trial. Circulation. 2018;137(20):2179–83.
White WB, Kupfer S, Zannad F, et al. Cardiovascular mortality in patients with type 2 diabetes and recent acute coronary syndromes from the EXAMINE trial. Diabetes Care. 2016;39(7):1267–73.
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003;63:225–32.
The American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S98-s110.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–93.
Drug and Medical Supply Information Center. 2021. 22 November 2021. http://dmsic.moph.go.th. Accessed 22 Nov 2021.
Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S17-26.
Anukoolsawat P, Sritara P, Teerawattananon Y. Costs of lifetime acute coronary syndrome treatment at Ramathibodi hospital. Thai Heart J. 2006;19:132–43.
Phrommintikul A, Dilokthornsakul P, Permsuwan U. Economic burdens for treatment of patients with type 2 diabetes in north Thailand: a hospital-based observational study. Front Endocrinol (Lausanne). 2022;13: 824545.
Bureau of Trade and Economics Indices, Ministry of Commerce. CPI 2020. http://www.price.moc.go.th/default5.aspx. Accessed 22 Nov 2021.
Kranenburg G, van der Graaf Y, van der Leeuw J, et al. The relation between HbA1c and cardiovascular events in patients with type 2 diabetes with and without vascular disease. Diabetes Care. 2015;38(10):1930–6.
Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thai. 2014;97(Suppl 5):S50–8.
Shah D, Risebrough NA, Perdrizet J, Iyer NN, Gamble C, Dang-Tan T. Cost-effectiveness and budget impact of liraglutide in type 2 diabetes patients with elevated cardiovascular risk: a US-managed care perspective. Clinicoecon Outcomes Res. 2018;10:791–803.
Deerochanawong C, Vareesangthip K, Piyayotai D, Thongsuk D, Pojchaijongdee N, Permsuwan U. Cost-utility analysis of dapagliflozin as an add-on to standard treatment for patients with type 2 diabetes and high risk of cardiovascular disease in Thailand. Diabetes Ther. 2021;12(7):1947–63.
Institute of Medical Research and Technology Assessment, Department of Medical Services, Ministry of Public Health. Economic evaluation of laparoscopic cholecystectomy versus open cholecystectomy 2020. Accessed 1 Feb 2022.
Pattanaprateep O, Ingsathit A, McEvoy M, Attia J, Thakkinstian A. Cost-effectiveness analysis of renin-angiotensin aldosterone system blockade in progression of chronic kidney disease. Value Health Reg Issues. 2018;15:155–60.
Permsuwan U, Dilokthornsakul P, Thavorn K, Saokaew S, Chaiyakunapruk N. Cost-effectiveness of dipeptidyl peptidase-4 inhibitor monotherapy versus sulfonylurea monotherapy for people with type 2 diabetes and chronic kidney disease in Thailand. J Med Econ. 2017;20(2):171–81.
The Nephrology Society of Thailand. Thailand renal replacement therapy: year 2016–2019. Bangkok 2020. https://www.nephrothai.org/annual-report-thailand-renal-replacement-therapy-2016-2019/. Accessed 1 Feb 2022.
Srisubat A, Jiamjariyaporn T, Chanpitakkul M, et al. Cost-effectiveness of integrated care in patients with chronic kidney disease stage 3 and 4 compared with standard care in rural communities. J Department Med Serv. 2017;42(6):54–63.
Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–31.
Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52(11):2288–98.
Acknowledgements
Funding
The authors thank Novo Nordisk (Thailand) for funding this study and the journals Rapid Service Fee.
Medical Writing and Editorial Assistance
The manuscript was drafted by Unchalee Permsuwan, Chaicharn Deerochanawong, and Rungroj Krittayaphong. All authors have approved the final version of manuscript to be published. The authors would also like to acknowledge Dr Anil Dandu, MPharm, PhD from Novo Nordisk Global Service Centre, Bengaluru, India for his editorial assistance in developing this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were responsible for the conception and design of the study, and for the acquisition, analysis, and interpretation of data. Unchalee Permsuwan analyzed the data and drafted the manuscript. All authors contributed substantially to drafting and revising of the manuscript. All authors have reviewed and approved the final version of the manuscript for submission to Diabetes Therapy and agree to be accountable for its contents.
Disclosures
Chaicharn Deerochanawong received speaker’s bureau honorarium from AstraZeneca, Boehringer Ingelheim and Novo Nordisk, and involved in research with Abbott, Boehringer Ingelheim and Novo Nordisk. Rungroj Krittayaphong and Unchalee Permsuwan have nothing to disclose. Jack Garcia Uranga Romano and Nicolai A Rhee are employed by Novo Nordisk. Nicolai A Rhee also holds shares at Novo Nordisk.
Compliance with Ethics Guidelines
This article is been approved ethics committees and was performed in accordance with the Helsinki Declaration of 1964.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Deerochanawong, C., Krittayaphong, R., Romano, J.G.U. et al. Cost–Utility of Liraglutide Plus Standard of Care Versus Standard of Care in People with Type 2 Diabetes and Cardiovascular Risk in Thailand. Diabetes Ther 14, 531–552 (2023). https://doi.org/10.1007/s13300-023-01371-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-023-01371-y