Abstract
Aims
For people with type 2 diabetes (T2D) on intensive insulin therapy, the use of flash continuous glucose monitoring (“flash monitoring”) is associated with improved average glucose control and/or reduced hypoglycemic exposure. This study assessed the cost-effectiveness of flash monitoring versus traditional blood glucose monitoring (BGM) in people with T2D using intensive insulin in the United Kingdom (UK).
Methods
The IQVIA CORE Diabetes Model (IQVIA CDM; v9.0) was used to analyze the impact of flash monitoring versus BGM over a 40-year time horizon from the UK payer perspective. Model inputs included baseline characteristics, intervention effects, resource utilization, costs, and utilities, based on recently published literature and national databases. UK National Health Service reimbursed costs of flash monitoring and BGM were used. An intervention-related health utility was obtained from a time trade-off study. Alternative scenarios were explored to assess the impact of key assumptions on base case results.
Results
In base-case analysis, flash monitoring compared with BGM resulted in an incremental cost of £5781 and an additional 0.47 quality-adjusted life years (QALYs). This provides an incremental cost-effectiveness ratio (ICER) of £12,309/QALY. HbA1c and the intervention-related health utility were the key drivers of differentiation. All scenario analyses, including different discount rates, time horizons, effects on HbA1c and on the intervention-related health utility, as well as glycemic emergencies, generated ICERs of less than £20,000 per QALY.
Conclusions
The consistent results across base case and a range of scenario analyses indicate that long-term flash glucose monitoring use is cost-effective compared with BGM in a UK population of T2D on intensive insulin therapy based on updated clinical effects and a cost-effectiveness threshold of £20,000–30,000 per QALY.
Similar content being viewed by others
The high risk of many T2D complications has led to a large and growing economic burden for health systems. |
Various RCT and RWE studies have demonstrated that flash glucose monitoring is associated with improvements in glucose control in people with T2D using intensive insulin. |
While previous analyses demonstrated the cost-effectiveness of flash glucose monitoring in people with T2D using intensive insulin, the aim of this analysis was to further assess the cost-effectiveness of this technology in view of more recent clinical evidence. |
In the base case analysis, flash glucose monitoring resulted in an ICER of £12,309/QALY and all scenario analyses generated ICERs of less than £20,000 per QALY. |
These results indicate that using flash glucose monitoring in people with T2D on intensive insulin therapy is cost-effective and should be considered in this population. |
Introduction
Diabetes is a common condition affecting 415 million people worldwide [1]. Approximately 90% of individuals with this condition have type 2 diabetes mellitus (T2D), which has a prevalence of 6% in the United Kingdom (UK) [1]. Diabetes increases the risk of microvascular and macrovascular complications, all of which contribute to increased patient morbidity and mortality [2]. The high risk of many T2D-associated complications has led to a large and growing economic burden for health systems. It has been estimated that the treatment of diabetes accounts for about 10% of the National Health Service’s (NHS’s) yearly budget, equating to over £9 billion per year, [1] the majority of which is spent on diabetes-related complications.
Optimizing glycemic control lowers the risk of complications and associated impact on quality of life. According to National Institute for Health and Care Excellence (NICE) diabetes guidance (NG28), a glycated hemoglobin (HbA1c) target of 6.5–8.0% (48–64 mmol/mol) is recommended, depending on patient characteristics, history of hypoglycemia, and presence of complications [3]. This flexibility in HbA1c targets has been necessary to limit hypoglycemia, particularly in high-risk individuals [4, 5]; for people with a history of severe hypoglycemia, limited life expectancy, or advanced cardiovascular complications, a threshold of 8% (64 mmol/mol) may be appropriate [3].
To optimize glycemic control, frequent testing of blood glucose levels via self-monitoring of blood glucose or blood glucose monitoring (BGM) systems has been used, and is recommended by NICE for people with T2D requiring insulin therapy [3]. However, BGM only provides sporadic data and can be inconvenient to patients, leading to underutilization [6, 7].
Flash glucose monitoring consists of a glucose sensor worn by the individual which continuously monitors interstitial glucose; data are wirelessly transferred by scanning from the sensor to a handheld reader (or smartphone). The system is factory calibrated and does not require calibration by the user. Each sensor lasts for 14 days before a replacement is required. Studies have demonstrated the efficacy and effectiveness of flash monitoring in people with T2D using intensive insulin. The REPLACE trial (NCT02082184) is a 6-month multicenter randomized controlled trial (RCT) of the flash glucose monitoring system versus self-monitoring of blood glucose in adults with T2D using intensive insulin and HbA1c level of 7.5–12% (58–108 mmol/mol). This trial found that flash monitoring led to both significant and sustained reductions in hypoglycemic exposure with no safety concerns [8], a finding that was reinforced through extended follow-up [9]. A subsequent RCT in T2D using intensive insulin found that flash monitoring was associated with an HbA1c improvement of − 0.53% (approximately 6 mmol/mol) compared with BGM with no increase in hypoglycemia and has also shown improved quality-of-life measures [10]. Additionally, real-world data from European countries have documented that flash monitoring users experience an HbA1c reduction of 0.9% (approximately 10 mmol/mol) on average over the course of 3–6 months [11], and, separately, in hypoglycemia and diabetic ketoacidosis [12].
While previous work demonstrated the cost-effectiveness of flash monitoring in people with T2D who are managed with intensive insulin [13,14,15], those analyses were based on a single RCT, which could be a limitation on the generalizability of the results. Therefore, our aim was to further assess the cost-effectiveness of flash glucose monitoring in view of recent clinical evidence, including both interventional and observational real-world studies, and explore the use of the device in T2D individuals from the UK health system perspective.
Methods
This study is a cost analysis and it did not involve any studies with human or animal subjects performed by any of the authors, hence there was no requirement to seek ethics committee approval.
IQVIA Core Diabetes Model
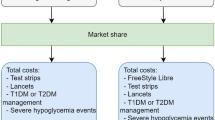
Cost-effectiveness analysis was performed using version 9.0 of the IQVIA Core Diabetes Model (IQVIA CDM). The model is designed to assess the long-term economic consequences and the lifetime health outcomes of interventions for type 1 and type 2 diabetes. The CDM has been extensively validated against results from clinical and epidemiological studies [16, 17]. It is a non-product-specific computer simulation model with disease progression based on a series of interdependent Markov submodels that simulate the complications of diabetes and non-specific mortality. Each submodel uses time, state, and diabetes type-dependent probabilities. Additionally, the submodels allow for patients to experience multiple events in the same 1-year cycle. These run at the same time in order to accurately capture comorbidities and outcomes associated with the treatment of interest. The simulations are performed at the patient level in order to allow interactions between the sub-models.
The model uses changes in physiological parameters associated with each treatment (e.g., HbA1c, lipids, blood pressure, body mass index) and translates them into changes in macrovascular and microvascular complications. Relevant risk equations were obtained from landmark studies such as the United Kingdom Prospective Diabetes Study (UKPDS) risk engine [18,19,20,21] and the Framingham Heart Study [22, 23]. Importantly, the model also includes a hypoglycemia module which considers severity (non-severe hypoglycemic event (NSHE), severe hypoglycemic event requiring third-party non-medical assistance (SHE1) and severe hypoglycemic event requiring third-party medical assistance (SHE2)) as well as (daytime) diurnal and nocturnal status. The cycle length is 4 months for SHE2 and daily for SHE1 and NSHEs.
Model outcomes include life expectancy, quality-adjusted life years (QALYs), costs, cumulative incidence of events such as complications and adverse events, as well as physiological outcomes such as HbA1c and BMI.
Model Inputs
The present study considered a payer perspective (NHS and Personal Social Service [PSS]) [24] with a lifetime horizon (assumed to be 40 years). In the base case, the time horizon was set to lifetime in order to capture all relevant long-term complications given the chronic nature of T2D, and to assess their impact on life expectancy, quality-adjusted life expectancy, and costs. Forty years aligns with the duration after which less than 0.5% of the cohort remains in the model. Discounting was set to 3.5% for both costs and effects based on NICE guideline recommendations [24]. All analyses were run with 1000 patients for 1000 iterations.
Baseline Characteristics
Baseline cohort characteristics used in the model were derived from the full analysis set population in the RCT by Yaron et al. [10] (Table 1). Some characteristics were unavailable in the study and therefore obtained from the REPLACE trial or, where UK-specific information was needed and details not available elsewhere, the UKPDS [8, 25]. At model entry, patients had a mean age of 67 years with mean duration of diabetes of 22 years. Mean HbA1c at baseline was 8.5%, reflecting the focus of the Yaron et al. trial on people with T2D whose HbA1c remains above 7.5% despite using intensive insulin therapy with regular BGM.
Intervention Effects
Intervention effects used in the analyses are presented in Table 2. The base case explored an incremental HbA1c benefit of 0.53% for flash glucose monitoring, based on Yaron et al.’s study [10]. The model also assumed that there are no other significant changes in physiological parameters in year 1, as these were not reported in the study. Changes in physiological parameters beyond year 1 were calculated based on progression data from the UK Prospective Diabetes Study (UKPDS) and Framingham studies [23, 25, 26].
The most common adverse event considered in the model was hypoglycemia, with event rates based on the literature. Severe and non-severe hypoglycemic events were evaluated separately. Since Yaron found no difference in hypoglycemic events, the incidence of severe hypoglycemic episodes was 105 (95% CI 0, 369) events per 100 patient-years (PY) for both arms [10, 27]. Non-severe events were assumed to reflect the mild/moderate hypoglycemic episodes at an incidence of 2331 events per 100 PY for both arms [27].
Intervention-Related Resource Utilization
Data regarding intervention-related resource utilization were obtained from a previously published study [13]. For the group using flash glucose monitoring, the study assumed use of 0.3 test strips and 0.69 lancets per day, and 85.2 units of insulin per day. In addition, this group would use 26 sensors per year and receive an additional physician visit in the first year. People using BGM were assumed to use three test strips per day, 1.26 lancets per day and 87.8 units of insulin per day. Both groups also received 1500 mg of metformin daily.
Costs
Table 3 shows the key cost inputs used in the present study, including intervention-related unit costs, total intervention costs, and direct costs for key acute events. All costs were reported in 2018 currency. Costs related to medications and procedures were derived from national databases. A complete cost list is available in the Supplementary Materials; costs related to complications were derived from published literature [28]. In the model, the NHS reimbursed price (£35.00) of the flash glucose monitoring sensor was used as the acquisition cost. The reader was assumed to incur no cost. The model included costs to the health care system only and does not capture costs to individuals. In addition, usage of flash monitoring and BGM were assumed to continue in perpetuity.
Utilities
Health benefits were expressed in terms of life years and QALYs gained. Key utility values are reported in Table 2. An intervention-related health utility benefit of 0.03 was applied to the flash monitoring arm based on the result of a published time-trade off (TTO) utility study [29]. Scenario analysis was done to evaluate the impact of varying health utility benefit on results. Other health state utilities and utility decrements were derived from the literature [30,31,32,33,34,35,36,37,38]. For NSHEs, the model employed a diminishing disutilities approach by leveraging the Lauridsen study [38]. This method reflects an updated understanding that disutility per event decreases as patients experience more events. Note, however, that disutilities for hypoglycemic events have limited impact on base case analysis, as this analysis leveraged observed differences in HbA1c, but no difference in hypoglycemia annual rates.
Scenario Analyses
The base case analysis was conducted to evaluate the cost-effectiveness of flash glucose monitoring versus BGM in people with T2D using intensive insulin in the UK using the default settings from Table 1. Additional scenario analyses were conducted to investigate the variability in results by changing the base case settings (Table 4). These include scenarios where an alternative discount rate of 0% and 6% was used and the time horizon was reduced to 5 years and 10 years instead of lifetime (base case), and lengthened (60 years) as well.
Furthermore, impact of utility benefit was tested according to 95% confidence interval (CI) values, HbA1c effect was varied by 20%, and alternate clinical effects from the literature were explored. The first alternate clinical effect was obtained from a meta-analysis conducted by Kröger et al., which found that flash monitoring decreased HbA1c levels by 0.90% (approximately 10 mmol/mol) in people with T2D with an average baseline HbA1c of 8.8% (73 mmol/mol) [11]. The scenario analysis used cohort data from Kröger where available [11], with REPLACE trial cohort for any missing cohort values [8]. Another clinical data scenario explored the impact to results if treatment effects solely reflect the RELIEF study [12], which found significant reductions in hypoglycemia and diabetic ketoacidosis (DKA) among people with T2D using intensive insulin after initiation of flash monitoring. The first intervention effect for this scenario was a 10.9% reduction in SHE2s; this reduction was applied to the base case rate of SHE2s rather than the annual percentage of events found in the RELIEF study, as the base rate from RELIEF study reflects hospitalizations only and not all third-party medical intervention. Additionally, a 52.1% reduction in DKA (annual probability of events of 0.82 vs. 1.7%) was implemented along with the hypoglycemia reduction [12]. Finally, although this study focuses primarily on clinical evidence for flash glucose monitoring beyond its original pivotal trials, a final scenario includes the reduction in NSHEs from the original REPLACE clinical trial for the sake of comprehensive comparison [8].
Results
Base Case
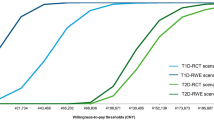
In base case (Yaron et al. [10]) using an HbA1c effect of − 0.53% associated with flash glucose monitoring compared with BGM, the total direct medical costs for flash monitoring and BGM use were £72,867 and £67,086, respectively, resulting in an incremental cost of £5781 attributable to flash monitoring. Cost differences reflect intervention costs offset by reduced complications, especially microvascular complications such as microalbuminuria, end-stage renal disease, and proliferative diabetic retinopathy. QALYs for flash monitoring over a lifetime horizon were estimated to be 4.73 vs. 4.26 when BGM was used, resulting in 0.47 additional QALYs for the flash monitoring group. The incremental cost-effectiveness ratio (ICER) was £12,309 per QALY gained for flash monitoring compared to BGM (Fig. 1a; Supplementary Material Table S1).
Scenario Analyses
The scenario based on Kröger et al. [11] in which clinical effectiveness showed a − 0.90% change in HbA1c (scenario 9) led to the lowest ICER, at £5821/QALY gained. The total direct medical costs for flash monitoring and BGM use were £81,255 and £77,630 respectively, resulting in an incremental cost of £3624, while incremental QALYs were 0.62, based on 5.07 and 4.45 for flash monitoring and BGM in this scenario. Conversely, the highest ICER, at £18,983, was associated with the shortest time horizon. This is related to shorter time over which the HbA1c benefit can reduce downstream complications, which would otherwise lead to greater cost offsets for flash glucose monitoring. Exploring alternate effectiveness in terms of HbA1c and utility benefit had a limited impact on ICER results, with all ICERs under £20,000/QALY (Fig. 1a). Additionally, considering only those scenarios with impact on intervention effects based on variation around the base-case analysis, alternate real-world data sources, and the original REPLACE clinical trial (Fig. 1b), all ICERs remain under £15,000.
Discussion
This study demonstrated that in people with T2D using intensive insulin therapy, use of a flash glucose monitoring was cost-effective compared to BGM when extrapolating effects from the range of clinical findings available at time of analysis. Although the overall cost associated with BGM is low, flash monitoring improves effect outcomes (QALYs) for people with T2D who are using intensive insulin, resulting in a favorable cost-effectiveness ratio in base-case analysis as well as alternate scenarios. Although alternate treatment effects impacted the magnitude of results, the model was most sensitive to the time over which intervention effects could impact occurrence of costly downstream diabetes-related complications. Despite variation, however, ICER values remained below accepted cost-effectiveness thresholds (typically about £30,000/QALY for the UK) across all scenarios explored. It should be noted that flash glucose monitoring has additional benefits by improving patient-related outcomes but these measures are not usually incorporated into health economic analyses.
This study translated clinical findings from available studies across different populations and in real-world use to understand the breadth of potential economic value compared to standard of care. The results of this study are in accordance with prior cost-effectiveness studies using original REPLACE trial data in a T2D population with intensive insulin use in a Swedish setting [13] as well as in several other European countries (Germany, Spain, Italy, France, Portugal, and the Netherlands) [14]. The primary intervention effect explored in those studies was a reduction in hypoglycemia along with health utility benefit, with the overall finding that flash monitoring had a modest impact on diabetes-related costs and favorable ICERs in the base case and all scenarios [13]. This study now leverages additional evidence that in populations where an HbA1c benefit occurs, flash glucose monitoring is cost-effective across a range of potential clinical effects in intensive insulin-treated people with T2D.
This work has a number of strengths. This is the first assessment to utilize data from different studies, including RCTs and real-world evidence, in a wide-ranging analysis of various scenarios. In particular, published efficacy or effectiveness results from each study are used individually in separate modeled scenarios, rather than making assumptions about potential combined benefits (e.g., HbA1c improvement concurrent with hypoglycemia reductions, when results are from separate research efforts). With consistent findings regardless of the included clinical studies, the conclusions are clear. Finally, the inclusion of different time horizons helps in translating the impact of study findings at various points of interest for the T2D intensive insulin-treated population.
As with any modeling study, there are some limitations. First, this analysis is unable to take into account potential cost savings associated with remote monitoring, which are facilitated by flash glucose monitoring. Remote access to comprehensive glucose data can reduce clinic attendance and make consultation more efficient, allowing introduction of treatment changes safely and effectively, which is not possible with BGM monitoring. This is particularly relevant with the recent COVID-19 pandemic, which led to the introduction of remote reviews for the majority of people with diabetes in the UK.
Second, the base-case analysis includes a value identified via a time tradeoff (TTO) study that assumed BGM users test their blood three times per day. NICE guidelines acknowledge BGM as appropriate for people receiving intensive insulin, and while no frequency is noted, other guidelines have suggested testing as often as 4–8 or 6–10 times per day [39]. Scenario analysis with the 95% CIs was performed to determine the impact of this type of utility benefit on final results, showing favorable results across the range.
Third, the work does not address patient-reported outcome measures (PROMs). While the current study considers the improved experience due to avoiding finger-sticks with BGM, other factors such as reduced anxiety related to hypoglycemic events were not included and could further impact results. Unfortunately, PROMs may not be considered in guidelines or when funding decisions are made on new glucose monitoring. It is possible that the improved PROMs translate clinically into better patient engagement, which is key in chronic conditions, and future work in this area is needed.
In addition, the mean age of the cohort for this analysis (67 years) and the mean duration of diabetes (22 years) was relatively old with a long duration of diabetes. However, the previously published CDM for the T2 population using intensive insulin, based on the REPLACE study, had a younger population (59 years) with a shorter diabetes duration (17 years). Combining the data, we believe the findings support the cost-effectiveness of flash monitoring in the entire population of people with T2D on intensive insulin therapy.
Finally, this analysis assumed that patients stayed on their intervention for the duration of the model, and that insulin dosing remained constant. It is a standard practice in modeling studies to assume that patients continue on an intervention consistently over the model time horizon; in this case, the implication is that any changes in insulin dose would impact both arms, thereby having little expected impact on projected health and economic consequences. Additionally, in this study, hypoglycemic event rates associated with flash glucose monitoring were assumed to be the same as for BGM due to lack of reported effect on hypoglycemia in the recent publications showing HbA1c improvement. It can be argued, however, that we were too cautious in our analysis, as flash monitoring has also previously been shown to offset hypoglycemic events in those with similar reduction in HbA1c compared with BGM [8]. Therefore, the current analysis may under-represent the full benefit of flash monitoring.
Conclusions
Taken together, these consistent data indicate that using flash glucose monitoring in people with T2D on intensive insulin therapy is cost-effective and should be considered in this population. The analysis likely underestimates the real economic impact of such a glucose monitoring strategy given some of the acknowledged limitations. In particular, remote glucose monitoring has the potential to result in significant cost savings, while reduced anxiety and improved PROMs may influence patient engagement thus improving long-term outcomes. Therefore, consideration should be given to expanding the funding of flash monitoring to people with T2D requiring intensive insulin therapy in the UK.
References
Diabetes Prevalence. 2019. (Accessed 27 Dec, 2019, at https://www.diabetes.co.uk/diabetes-prevalence.html).
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88.
Type 2 diabetes in adults: management (NG28). 2015. (Accessed Apr, 2018, at https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#initial-drug-treatment).
Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347: f4533.
Pearson SM, Whittam B, Kulavarasalingam K, Mitchell-Gears A, James C, Ajjan RA. Reduction in cardiovascular mortality following severe hypoglycemia in individuals with type 2 diabetes: the role of a pragmatic and structured intervention: structured intervention for community hypoglycemia. Cardiovasc Diabetol. 2021;20:18.
Fisher WA, Kohut T, Schachner H, Stenger P. Understanding self-monitoring of blood glucose among individuals with type 1 and type 2 diabetes: an information-motivation-behavioral skills analysis. Diabetes Educ. 2011;37:85–94.
Wagner J, Malchoff C, Abbott G. Invasiveness as a barrier to self-monitoring of blood glucose in diabetes. Diabetes Technol Ther. 2005;7:612–9.
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55–73.
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Use of flash glucose-sensing technology for 12 months as a replacement for blood glucose monitoring in insulin-treated Type 2 diabetes. Diabetes Ther. 2017;8:573–86.
Yaron M, Roitman E, Aharon-Hananel G, et al. Effect of flash glucose monitoring technology on Glycemic control and treatment satisfaction in patients with Type 2 diabetes. Diabetes Care. 2019;42:1178–84.
Kroger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with Type 2 diabetes. Diabetes Ther. 2019. https://doi.org/10.2337/db19-99-LB.
Roussel R, Riveline JP, Vicaut E, et al. Important drop rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study. Diabetes Care 2021.
Bilir SP, Hellmund R, Wehler E, Li H, Munakata J, Lamotte M. The cost-effectiveness of a flash glucose monitoring system for management of patients with type 2 diabetes receiving intensive insulin treatment in Sweden. Eur Endocrinol. 2018;14:80–5.
Li H, Bilir SP, Wehler EA, Hellmund R, Munakata J. Cost effectiveness analysis of a flash glucose monitoring system for type 2 diabetes (T2DM) patients receiving intensive insulin treatment in Europe. Value Health. 2016;19:A698.
Jendle J, Eeg-Olofsson K, Svensson A-M, Franzen S, Lamotte M, Levrat-Guillen F. Cost-effectiveness of the FreeStyle Libre® system versus blood glucose self-monitoring in individuals with type 2 diabetes on insulin treatment in Sweden. Diabetes Ther. 2021;12:3137–52.
Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(Suppl 1):S5-26.
McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE diabetes model. Value Health. 2014;17:714–24.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Stevens RJ, Kothari V, Adler AI, Stratton IM, United Kingdom Prospective Diabetes Study G. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101:671–9.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Kothari V, Stevens RJ, Adler AI, et al. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33:1776–81.
Wilson PW, Evans JC. Coronary artery disease prediction. Am J Hypertens. 1993;6:309S-S313.
Wilson PW, Kannel WB. Hypercholesterolemia and coronary risk in the elderly: the Framingham study. Am J Geriatr Cardiol. 1993;2:56.
National Institute of Health and Care Excellence - Guide to the methods of technology appraisal 2013 (PMG9). 2013. (Accessed Apr, 2018, at https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781).
Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33.
Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia. 2004;47:1747–59.
Edridge CL, Dunkley AJ, Bodicoat DH, et al. Prevalence and incidence of Hypoglycaemia in 532,542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta-analysis of population based studies. PLoS One. 2015;10: e0126427.
Hammer M, Lammert M, Mejías SM, Kern W, Frier BM. Costs of managing severe hypoglycaemia in three European countries. J Med Econ. 2009;12:281–90.
Matza LS, Stewart KD, Davies EW, Hellmund R, Polonsky WH, Kerr D. Health state utilities associated with glucose monitoring devices. Value Health. 2017;20:507–11.
Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22:340–9.
Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14:217–30.
Wasserfallen JB, Halabi G, Saudan P, et al. Quality of life on chronic dialysis: comparison between haemodialysis and peritoneal dialysis. Nephrol Dial Transplant. 2004;19:1594–9.
Kiberd BA, Jindal KK. Screening to prevent renal failure in insulin dependent diabetic patients: an economic evaluation. BMJ. 1995;311:1595–9.
Fenwick EK, Xie J, Ratcliffe J, et al. The impact of diabetic retinopathy and diabetic macular Edema on health-related quality of life in type 1 and type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53:677–84.
Lee WJ, Song KH, Noh JH, Choi YJ, Jo MW. Health-related quality of life using the EuroQol 5D questionnaire in Korean patients with type 2 diabetes. J Korean Med Sci. 2012;27:255–60.
Marrett E, Radican L, Davies MJ, Zhang Q. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes. 2011;4:251.
Evans M, Khunti K, Mamdani M, et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11:90.
Lauridsen JT, Lonborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23:2645–50.
American Diabetes A. 6. Glycemic targets: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S55–64.
Acknowledgements
Funding
This research and journal’s Rapid Service Fee was funded by Abbott Diabetes Care.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
RAA contributed to the design of the work, validated the medical inputs for the model and critically reviewed the draft manuscript; SPB developed and performed the analyses, drafted and reviewed the manuscript; DS and RH contributed to planning and reviewing the analyses, and reviewed and contributed to the draft manuscript.
Disclosures
Ramzi A. Ajjan received honoraria and research support from Abbott Diabetes Care, unrelated to this work. S, Pinar Bilir is an employee of IQVIA, which was hired to perform the analyses. No direct payment was received. Diana Souto was formerly an employee of Abbott Diabetes Care and is a stockholder; she is now an employee of Gilead Sciences. Richard Hellmund is a contracted employee of Abbott Diabetes Care and is a stockholder.
Compliance with Ethics Guidelines
This study is a cost analysis. It did not involve any studies with human or animal subjects performed by any of the authors, hence there was no requirement to seek ethics committee approval.
Data Availability
All data generated or analyzed during this study are included in this published article or as Supplementary Information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ajjan, R., Bilir, S.P., Hellmund, R. et al. Cost-Effectiveness Analysis of Flash Glucose Monitoring System for People with Type 2 Diabetes Receiving Intensive Insulin Treatment. Diabetes Ther 13, 1933–1945 (2022). https://doi.org/10.1007/s13300-022-01325-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01325-w