Abstract
Heart failure (HF) and chronic kidney disease (CKD) are the most frequent first cardiorenal conditions in patients with type 2 diabetes (T2D), which can be exacerbated by other comorbidities, such as hypertension, dyslipidemia, and obesity. To improve their clinical outcomes, patients with T2D need to achieve and maintain glycemic targets, as well as prevent cardiorenal disease onset and progression. Several clinical trials evaluating the sodium–glucose cotransporter type 2 inhibitors (SGLT2i) dapagliflozin, empagliflozin, canagliflozin, and ertugliflozin have shown consistent risk reduction in major adverse cardiovascular events and/or hospitalization for HF, together with lower risk of kidney disease progression. The benefits associated with SGLT2i in T2D are distinct from other antihyperglycemic drugs since they have been proposed to exert pleiotropic metabolic and direct effects on the kidney and the heart. In this review, we summarize and discuss the evidence regarding the mechanisms of action, the efficacy and safety profiles, and the clinical guidelines on the use of the therapeutic class of SGLT2i, highlighting their role in cardiorenal prevention beyond glycemic control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
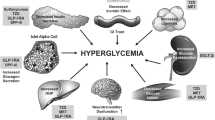
Patients with type 2 diabetes (T2D) often experience cardiovascular and renal complications mainly due to chronic hyperglycemia. |
There is a need for effective and well-tolerated treatments that may help patients with T2D achieve and maintain glycemic control, as well as prevent cardiorenal disease onset and progression. |
Sodium–glucose cotransporter type 2 inhibitors (SGLT2i) facilitate urine glucose and sodium excretion. The increased glycosuria and natriuresis underlie their metabolic benefits, such as a reduction in glycosylated hemoglobin, body weight, and blood pressure. The benefits of SGLT2i have expanded beyond their glucose-lowering effect, as these agents have proven to exert pleiotropic metabolic and direct effects on the kidney and the heart. |
Additionally, several placebo-controlled clinical trials evaluating SGLT2i have shown consistent risk reductions in adverse cardiovascular and renal events. |
This evidence is promoting a change of treatment paradigm to a more comprehensive approach of T2D that goes beyond glycemic control, also focusing on the prevention and delaying of cardiorenal complications. |
Introduction
Diabetes-related disease burden and economic impact are mainly due to comorbidities and complications, such as heart failure (HF), atherosclerotic cardiovascular disease (CVD), chronic kidney disease (CKD), neuropathy, and retinopathy [1]. HF and CKD have been shown to be the most frequent first cardiorenal manifestations in patients with type 2 diabetes (T2D) initially free of cardiorenal diseases, as the events also associated with increased risk of further CVD and mortality [2]. This high cardiorenal risk is the result of chronic hyperglycemia and is exacerbated by other comorbidities, such as hypertension, dyslipidemia, and obesity. Thus, there is a need for effective and well-tolerated treatments that may help patients with T2D achieve and maintain glycemic control, as well as prevent cardiorenal disease onset and progression. Among the different classes of glucose-lowering agents, the sodium–glucose cotransporter type 2 inhibitors (SGLT2i) have shown potential to address this need. Several clinical trials evaluating the SGLT2i empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin have shown consistent risk reduction in major adverse cardiovascular events and/or hospitalization for HF, as well as lower risk in kidney disease progression, versus placebo [2,4,5,6,6]. In this review, we aimed to summarize and discuss the evidence published in recent years in relation to the therapeutic class of SGLT2i and their role in cardiorenal prevention. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
SGLT2i and Their Unique Mechanism of Action in Metabolic Control
Glucose reabsorption in the kidney is mainly mediated by SGLT2 receptor, located in the early proximal tubule, with a small contribution of sodium–glucose cotransporter type 1 (SGLT1), located in the late proximal tubule [7]. All the SGLT2i share the same mechanism of action: by inhibiting SGLT2 receptor, they facilitate urine glucose and sodium excretion. Canagliflozin also exerts a small inhibiting effect on SGLT1 receptor [8], which contributes to the uptake of glucose and galactose in the intestine, but plays a minor role in renal reabsorption [9]. The increased glycosuria and natriuresis mediated by SGLT2 inhibition associate with different metabolic benefits, such as a reduction in glycosylated hemoglobin (HbA1c), body weight, and blood pressure [10]. The renal action of SGLT2i is independent of β‐cell function and insulin secretion, limiting the risk of hypoglycemia; thus, these drugs have the potential to be used at any stage of T2D and in combination with any class of glucose‐lowering agent, including insulin [10], although their glucosuria effect is lower when renal function declines.
Current SGLT2i approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of patients with T2D are dapagliflozin, empagliflozin, canagliflozin, and ertugliflozin. Generally, SGLT2i seems to be as effective at decreasing plasma glucose levels as other available therapeutic options [11]. Although there are real-life studies that suggest differences between SGLT2i [12], head-to-head comparisons between the different gliflozins are lacking, so it is unknown if any improve glycemic control more than the others. A network meta-analysis showed that SGLT2i as monotherapy were more effective than placebo for lowering HbA1c levels (by 0.59–1.23%) after 24 weeks of treatment [13]. Similarly, in another meta-analysis that included trials evaluating dapagliflozin, this drug produced greater mean reductions in HbA1c (−0.60%), fasting plasma glucose (−1.30 mmol/L), and body weight (−1.50 kg), compared with placebo [14]. Apart from these metabolic benefits, SGLT2i have demonstrated to exert direct nephroprotective and cardioprotective effects [15] and to be associated with favorable CV and kidney outcomes [16]. This evidence is promoting a change of treatment paradigm, shifting from targeting glucose control and HbA1c concentrations to reduce microvascular complications, to a more comprehensive approach that also focuses on CV and renal prevention [17].
Clinical Evidence of Cardiorenal Protection in SGLT2i-Treated Patients
Classically, the clinical development of antidiabetic agents focused on glucose-lowering effects only. However, after the rosiglitazone controversy [18] and the discovery that oral hypoglycemic drugs can maintain a normal blood glucose level but paradoxically increase CV events in patients with T2D [19], in 2008 the FDA issued a guidance for industry to include CV safety as an endpoint for all novel antihyperglycemic medications [20]. Since then, several antidiabetic agents have undergone randomized placebo-controlled cardiovascular outcome trials (CVOTs), which mainly recruited patients with preexisting CVD or a high risk of developing it [18].
To date, four main studies have evaluated the CV outcomes of empagliflozin, canagliflozin, dapagliflozin, and ertugliflozin in patients with T2D and diverse baseline CV risk profiles (Table 1). The EMPA-REG OUTCOME trial (Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes) examined the efficacy of empagliflozin in 7020 patients with T2D and established CVD (secondary prevention) [3]. It was the first study to demonstrate CV efficacy, with a 14% reduction in the primary composite outcome of MACE (3-point MACE; CVD death, myocardial infarction, and stroke). Regarding secondary outcomes, it showed a 32% reduction in all-cause mortality, 38% in cardiovascular mortality, and 32% in hospitalization for HF (Table 1). Likewise, the kidney composite outcome of albuminuria progression, doubling of serum creatinine, end-stage kidney disease (ESKD), or renal death was reduced by 39%, with benefits also evident in patients without CKD at baseline [21].
The CANVAS PROGRAM (Canagliflozin Cardiovascular Assessment Study) comprised two clinical trials that tested the efficacy of canagliflozin in 10,142 patients, of which 65.6% had a history of CVD (secondary prevention) and the remaining had no known CVD (primary prevention); 20.1% of participants had eGFR < 60 ml/min/1.73 m2 [4]. There was a 14% reduction in the 3-point MACE primary outcome, as well as a 33% reduction in hospitalization for HF (secondary outcome). No significant reduction in all-cause mortality was achieved (Table 1). There was a 40% reduction of the prespecified composite renal (secondary) outcome in participants using canagliflozin compared with those using placebo. Moreover, canagliflozin was associated with a 47% reduction in doubling of serum creatinine, ESKD, and death due to kidney disease [22].
The DECLARE-TIMI 58 trial (Dapagliflozin Effect on Cardiovascular Events-Thrombolysis In Myocardial Infarction 58) was the largest study of the four CVOTs of SGLT2i, with 17,160 patients included, 59% of whom did not have established CVD (primary prevention) [5]. The overall effect of dapagliflozin on the 3-point MACE coprimary outcome was not statistically significantly different versus placebo, although the combined coprimary outcome of CV mortality and hospitalization for HF was significantly reduced [hazard ratio 0.83, 95% confidence interval (CI) 0.73–0.95], which reflected a lower rate of hospitalization for HF (hazard ratio 0.73, 95% CI 0.61–0.88) (Table 1). Compared with placebo, dapagliflozin was associated with a 47% reduction in the composite renal secondary outcome (40% decline in eGFR, ESKD or renal death) [23]. Subsequent post-hoc analyses showed that, in patients with previous myocardial infarction, dapagliflozin reduced the risk of MACE by 16% and cardiovascular death/hospitalization for HF by 19% [24], whereas it decreased the rate of new hospitalizations for HF in patients with established HF and cardiovascular death and all-cause mortality in patients with HF with reduced ejection fraction [25].
The VERTIS-CV study (Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants with Vascular Disease) compared ertugliflozin versus placebo in 8,252 participants with T2D and established atherosclerotic CVD; at baseline, 22.0% of participants had eGFR < 60 ml/min/1.73 m2, and 9.0% of participants had macroalbuminuria [6]. Ertugliflozin showed no significant difference with regard to the primary 3-point MACE outcome, but it was associated with a 30% reduction in hospitalization for HF (Table 1). For the key composite kidney endpoint, a trend was noted in terms of beneficial effect, although this was not statistically significant. Subgroup analysis suggested a benefit in hospitalization for HF/CV death with ertugliflozin versus placebo among patients with more advanced renal disease [26].
Thus, these large international CVOTs demonstrated the CV and renal favorable effects of SGLT2i, which have been confirmed in recent meta-analyses [16, 27], indicating that drugs in this class reduced the 3-point MACE, as well as hospitalization for HF and onset or progression of kidney disease. The reduction in hospitalization for HF and related outcomes was the most consistent effect, independent of baseline atherosclerotic CVD and prior HF and across the spectrum of baseline kidney function. However, some heterogeneity was detected in the associations with outcomes of different SGLT2i on CV death, possibly due to differences in the populations studied and their risk profiles [16]. Moreover, the trials evaluating empagliflozin and ertugliflozin enrolled 100% of patients with established CVD, focusing on the secondary prevention, whereas the trials evaluating canagliflozin and dapagliflozin enrolled patients both with and without CVD. In the final combined dataset, the majority of patients had prevalent CVD, so the effects of SGLT2i in CV primary prevention have been primarily studied and demonstrated just for dapagliflozin and, to a minor extent, canagliflozin [28].
The highly selected and elevated proportion of patients with established CVD/high CV risk enrolled in the CVOTs could limit the generalizability of the results to the real-world setting. An investigation of how the four different SGLT2i CVOT populations, according to their respective selection criteria, were representative of general T2D populations in four European countries showed that real-world patients had less prevalent CVD and were slightly older than those recruited in the CVOTs. The DECLARE‐TIMI 58 trial had the highest representativeness, covering 59% of the general T2D population, whereas this proportion was 34% in CANVAS, 21% in EMPA‐REG OUTCOME, and 17% in VERTIS‐CV [29]. Even so, the CVD-REAL studies, which compared CV and renal outcomes in > 400,000 propensity-matched patients with T2D who newly initiated SGLT2i or other glucose-lowering therapy in different countries, found that SGLT2i were associated with significantly lower risks of all-cause mortality, hospitalization for HF, and major kidney events, compared with other glucose-lowering drugs such as dipeptidyl peptidase-4 inhibitors [30,31,32,33]. In general, the risk of CV outcomes decreased not only in patients with established CVD but also in those without CVD at baseline [32].
Safety Profile of SGLT2i
The four gliflozins currently marketed are all orally bioavailable and have similar half-life [34]. The results of the dedicated CVOTs of SGLT2i (EMPA-REG OUTCOME with empagliflozin, CANVAS with canagliflozin, DECLARE-TIMI 58 with dapagliflozin, and VERTIS-CV with ertugliflozin) consistently excluded an excess risk of CV events in patients with long-standing T2D and established CVD or multiple CV risk factors [3,4,5,6]. Moreover, many of the participants had impaired kidney function (baseline range for eGFR, 30 to ≥ 90 mL/min/1.73 m2; baseline range for albuminuria, UACR < 30 to > 300 mg/g), and the reduction of CV adverse events was similar to the observed in the overall trial populations. In two dedicated renal outcomes trials (CREDENCE with canagliflozin and DAPA-CKD with dapagliflozin) that included patients with CKD, the risk of kidney failure and CV events decreased compared with placebo [35, 36].
Regarding noncardiorenal safety, SGLT2i generally have a favorable tolerability profile (Table 2). Serious adverse events, hypoglycemia, and acute kidney injury are less common among patients receiving SGLT2i than controls [37]. In fact, the adverse events reported in clinical trials are mostly associated with their main mechanism of action that provokes increased glycosuria and diuresis. This is the case for genitourinary tract infections, which are the most frequent adverse events reported across trials [38]. However, these episodes are usually mild to moderate and rarely require discontinuation of the medication. There is also a higher risk of volume depletion-related events due to the effect of osmotic diuresis of glucose and sodium [37], particularly in older patients and those receiving concomitant diuretic therapy, which should be adjusted before starting treatment with SGLT2i. Although rare [a recent meta-analysis reported only 56 events in 30,766 individuals receiving canagliflozin, dapagliflozin, or empagliflozin (0.18%) versus control: 23 in 25,211 (0.09%)] [39], diabetic ketoacidosis with minimal to no elevation of glycemia (euglycemic ketoacidosis) may occur in patients taking SGLT2i. Anticipatory guidance to pause SGLT2i dosing during periods of acute illness or other stressors may limit this adverse effect [40]. Higher rates of lower extremity amputation were reported for canagliflozin versus placebo in the CANVAS study [4], but this heightened risk has not been observed in clinical trials evaluating other SGLT2i [38] or with canagliflozin in the CREDENCE trial [35]. Performing frequent foot examinations and interrupting the therapy during active ulcerations or foot lesions, which were the measures implemented during the CREDENCE trial, may decrease amputation rates.
Current Knowledge on the Mechanisms Underlying the Cardiorenal Prevention Observed with SGLT2i
As stated above, the molecular effects and clinical benefits exerted by SGLT2i in T2D are distinct from other antihyperglycemic drugs. The benefits of gliflozins have expanded beyond their glucose-lowering effect, and these agents have proven to exert pleiotropic metabolic and direct effects on the kidney and the heart [41]. Firstly, they seem to initiate a series of metabolic adaptations that arise in response to the caloric deficit caused by the increased glucosuria, together with the reduction of glucotoxicity. This may prevent oxidative stress, endothelial dysfunction, inflammation, and fibrosis in multiple targeted tissues (kidney, arteries, retina, heart, adipose tissue). The inhibition of proximal tubular sodium and glucose reabsorption increases distal sodium delivery to the macula densa and restore tubule–glomerular feedback, resulting in lower intraglomerular pressure, shear stress, hyperfiltration, and albuminuria [30, 42]. On the other hand, the osmotic diuresis and plasma volume reduction, and the decrease in blood pressure and arterial stiffness, are supposed to improve cardiac preload and afterload, decreasing oxygen consumption, which could benefit patients with heart failure [43]. The increase in hematocrit levels provides more oxygen to the heart muscle. This increase could result from the reduction of plasma volume and/or the augmentation of erythropoietin synthesis [44]. Additionally, SGLT2i reduce body weight and uric acid, contributing to the wide arsenal of cardiometabolic benefits [15].
However, the effects of improved glycemic control, diuresis, and weight and blood pressure reductions do not fully explain the observed benefits in cardiorenal outcomes, and increasing evidence indicates that the cardiac and renal protection provided by SGLT2i is related to other “nonconventional” mechanisms [43] (see Fig. 1 in the article dedicated to heart failure in this supplement). For example, glucosuria enhances gluconeogenesis and ketogenesis, probably by the activation of sirtuin-1, which induce a shift to a “fasting state” [45]. It is hypothesized that the increase in the synthesis of ketone bodies changes the main fuel of heart tissue to β-hydroxybutyrate, instead of fatty acids, which is considered a “super fuel” that improves myocardial energy dynamics and exerts direct antiinflammatory effects [46]. In animal models, SGLT2i decreases cardiac cytosolic sodium content by blocking the sodium–hydrogen exchanger (NHE) isoform 1 in myocytes, thereby reducing intracellular Na+ and Ca2+ concentrations and increasing mitochondrial Ca2+. As calcium overload results in dysfunction and loss of cardiomyocytes, SGLT2i may improve the myocardial electrochemical characteristics, which potentially contribute to their CV benefit [47]. Furthermore, SGLT2i prevent adipose tissue hypertrophy caused by a high-fat diet, improving its secretory profile (↑ adiponectin, ↓ leptin). This process produces a CV benefit by reducing the leptin in the body, which has been linked to high blood pressure and other CV risk factors [48].
SGLT2i Positioning in Current Society Guidelines
As a result of the favorable results observed in the CVOTs and real-world studies, major national and international professional societies have updated their recommendations and guidelines to prioritize the use of SGLT2i, regardless of glucose control considerations, in patients with T2D with or at high risk for CV and kidney complications (Table 3). Current American Diabetes Association (ADA) guidelines state that first-line therapy depends on comorbidities, patient-centered treatment factors, and management needs and generally includes metformin and comprehensive lifestyle modification. Medications such as SGLT2i or glucagon-like peptide-1 receptor agonists (GLP-1 RA) are the recommended initial therapy for individuals with T2D with or at high risk for atherosclerotic CVD, HF (only SGLT2i), and/or CKD (preferably SGLT2i in those with CKD and albuminuria), independently of metformin use and baseline or target HbA1c levels [49]. By contrast, the European Society of Cardiology/European Association for the Study of Diabetes (ESC/EASD) guidelines recommend the use of SGLT2i or GLP-1 RA in all patients with T2D with established or high/very high risk for CVD, either in monotherapy for treatment-naïve patients or added to the background treatment, regardless of other considerations such as the status of the glycemic control [50]. For patients with atherosclerotic CVD, HF, and/or CKD, the Diabetes Canada Guidelines prioritize the use of agents with demonstrated CV or renal benefits (SGLT2i or GLP-1 RA), combined or not with metformin according to glycemic control and clinical status [51]. The Primary Care Diabetes Europe categorizes patients with T2D into different risk groups based on individual factors such as atherosclerotic CVD, HF, CKD, and obesity, and recommend that SGLT2i should be prioritized over GLP-1RA for patients with HF or CKD [52].
Conclusions and Perspective
Although SGLT2i were initially only considered as glucose-lowering agents, the effects of gliflozins have expanded far beyond that, thanks to their pleiotropic metabolic and direct nephroprotective and cardioprotective effects. These mechanisms of action of SGLT2i appear to have little connection with the underlying disease of T2D, and therefore their cardiorenal benefits also exist in patients without T2D. There is no doubt that prevention and treatment in early stages of T2D is more cost-effective than the management of more advanced and complicated disease [53]. In light of the favorable results obtained in the aforementioned studies, we should consider not only that we can treat patients with T2D and comorbidities better, but also that we already can avoid or delay these complications. Thus, the assessment of CVD and CKD risk status in every patient at their first presentation has become mandatory in order to individualize therapy according to the clinical status.
There are still some barriers that have prevented the guidelines recommendations from being translated into practice, such as the misconception that SGLT2is are not safe drugs. For example, one of the most common adverse effects is mycotic genital infections, but easy-to-implement hygienic measures have been shown to reduce the risk for this complication and improve compliance with SGLT2i therapy [54]. Moreover, the absolute risk increase in adverse effects seems to be small and acceptable when it is contrasted with the benefits [55]. Thus, it is of particular importance for primary and secondary care physicians to become familiar and comfortable with these agents—both their benefits and side effect profiles—and be willing to prescribe them. With regard to the generality of the results of the clinical trials, it should be taken into account that the majority of the participants were white, so more studies are needed in other ethnic or racial groups.
References
American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41:917–28.
Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22:1607–18.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–35.
Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94.
Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–61.
Danne T, Biester T, Kordonouri O. Combined SGLT1 and SGLT2 inhibitors and their role in diabetes care. Diabetes Technol Ther. 2018;20:S2-69-S2-77.
Scheen AJ. Sodium–glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16:556–77.
Jia S, Wang Z, Han R, et al. Incretin mimetics and sodium–glucose co-transporter 2 inhibitors as monotherapy or add-on to metformin for treatment of type 2 diabetes: a systematic review and network meta-analysis. Acta Diabetol. 2021;58:5–18.
Gorgojo-Martínez JJ, Gargallo-Fernández MA, Galdón Sanz-Pastor A, Antón-Bravo T, Brito-Sanfiel M, Wong-Cruz J. Real-world clinical outcomes associated with canagliflozin in patients with type 2 diabetes mellitus in Spain: the Real-Wecan study. J Clin Med. 2020;9:2275.
Shyangdan DS, Uthman OA, Waugh N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. BMJ Open. 2016;6:e009417–e009417.
Feng M, Lv H, Xu X, Wang J, Lyu W, Fu S. Efficacy and safety of dapagliflozin as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Medicine. 2019;98: e16575.
Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium–glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:422–34.
McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–58.
Ismail-Beigi F, Moghissi E, Kosiborod M, Inzucchi SE. Shifting paradigms in the medical management of type 2 diabetes: reflections on recent cardiovascular outcome trials. J Gen Intern Med. 2017;32:1044–51.
Hiatt WR, Kaul S, Smith RJ. The cardiovascular safety of diabetes drugs—insights from the rosiglitazone experience. N Engl J Med. 2013;369:1285–7.
Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–36.
US Food and Drug Administration. Guidance for Industry on Diabetes Mellitus-Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008. Available from: https://www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic. Accessed on 23 Sep 2021.
Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Perkovic V, de Zeeuw D, Mahaffey KW, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704.
Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–17.
Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139:2516–27.
Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–36.
Cherney DZI, McGuire DK, Charbonnel B, et al. Gradient of risk and associations with cardiovascular efficacy of ertugliflozin by measures of kidney function: observations from VERTIS CV. Circulation. 2021;143:602–5.
Arnott C, Li Q, Kang A, et al. Sodium–glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9: e014908.
Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events. Circulation. 2018;137:323–34.
Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21:968–74.
Heerspink HJL, Karasik A, Thuresson M, et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): a multinational observational cohort study. Lancet Diabetes Endocrinol. 2020;8:27–35.
Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium–glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium–glucose cotransporter-2 inhibitors). Circulation. 2017;136:249–59.
Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–39.
Real J, Vlacho B, Ortega E, et al. Cardiovascular and mortality benefits of sodium–glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus: CVD-Real Catalonia. Cardiovasc Diabetol. 2021;20:139.
Garcia-Ropero A, Badimon JJ, Santos-Gallego CG. The pharmacokinetics and pharmacodynamics of SGLT2 inhibitors for type 2 diabetes mellitus: the latest developments. Expert Opin Drug Metab Toxicol. 2018;14:1287–302.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–46.
Zhang X-L, Zhu Q-Q, Chen Y-H, et al. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc. 2018;7: e007165.
Pelletier R, Ng K, Alkabbani W, Labib Y, Mourad N, Gamble J-M. Adverse events associated with sodium glucose co-transporter 2 inhibitors: an overview of quantitative systematic reviews. Ther Adv Drug Saf. 2021;12:2042098621989134.
Liu J, Li L, Li S, et al. Sodium-glucose co-transporter-2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;22:1619–27.
Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor-associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis. Clin Ther. 2016;38:2654-2664.e1.
Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020;17:761–72.
Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018;94:26–39.
Joshi SS, Singh T, Newby DE, Singh J. Sodium–glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart. 2021;107:1032–8.
Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation. 2019;139:1985–7.
Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. 2020;43:508–11.
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–14.
Uthman L, Baartscheer A, Bleijlevens B, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61:722–6.
Wu P, Wen W, Li J, et al. Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes. Horm Metab Res. 2019;51:487–94.
American Diabetes Association. Standards of medical care in diabetes—2022 abridged for primary care providers. Clin Diabetes. 2022;40:10–38.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes. 2020;44:575–91.
Seidu S, Cos X, Brunton S, et al. A disease state approach to the pharmacological management of type 2 diabetes in primary care: a position statement by Primary Care Diabetes Europe. Prim Care Diabetes. 2021;15:31–51.
Herman WH. The cost-effectiveness of diabetes prevention: results from the diabetes prevention program and the diabetes prevention program outcomes study. Clin Diabetes Endocrinol. 2015;1:9.
Williams SM, Ahmed SH. Improving compliance with SGLT2 inhibitors by reducing the risk of genital mycotic infections: the outcomes of personal hygiene advice. Diabetes. 2019;68:1224.
Johansen ME, Argyropoulos C. The cardiovascular outcomes, heart failure and kidney disease trials tell that the time to use Sodium Glucose Cotransporter 2 inhibitors is now. Clin Cardiol. 2020;43:1376–87.
Acknowledgements
Funding
AstraZeneca funded the writing assistance provided by Springer Healthcare Ibérica SL. The journal’s Rapid Service Fee was funded by AstraZeneca.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors equally participated in the development of the manuscript, had full editorial control, reviewed and edited various drafts, and provided their final approval of all content and submission for publication.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Anabel Herrero, PhD, on behalf Springer Healthcare Ibérica and was funded by AstraZeneca. The authors want to express their gratitude to José Juan Aparicio-Sánchez from the Medical Affairs department of AstraZeneca Spain for his continuous scientific support during the development of this manuscript.
Disclosures
Manuel Botana has received speaking fees from AstraZeneca, Lilly, Boehringer, MSD, Esteve, NovoNordisk, Sanofi, Janssen, and Almirall; he has served as an advisor with Amgen, AstraZeneca, NovoNordisk, Merck, Novartis, and Daiichi-Sankyo. Javier Escalada has received fees as a speaker or consultant from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esteve, MSD and NovoNordisk; he has been an investigator in clinical trials for Eli Lilly and NovoNordisk. Ángel Merchante has received fees as a speaker or consultant from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Esteve, MSD, NovoNordisk and Sanofi. Rebeca Reyes has received fees as a speaker or consultant from NovoNordisk, Eli Lilly, Boehringer-Ingelheim, Astra Zeneca, MSD, Ascensia, Esteve, FAES, Almirall. Pedro Rozas has received fees as speaker or consultant from NovoNordisk, Eli Lilly, Boehringer-Ingelheim, Astra Zeneca, MSD, Esteve, FAES, Sanofi, Janssen, Novartis, and Daiichi-Sankyo.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Botana, M., Escalada, J., Merchante, Á. et al. Prevention of Cardiorenal Complications with Sodium–Glucose Cotransporter Type 2 Inhibitors: A Narrative Review. Diabetes Ther 13 (Suppl 1), 5–17 (2022). https://doi.org/10.1007/s13300-022-01277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01277-1