Abstract
Introduction
Sarcopenia is defined as a progressive and generalized muscle disorder that involves accelerated loss of muscle mass and impaired function. It is believed to influence the ability to carry out daily activities, muscle strength, and physical capacity in the elderly. Studies have shown that sarcopenia has been implicated as both a cause and a consequence of diabetes mellitus. In this analysis, we aimed to systematically show the impact of exercise intervention as a therapy for patients with diabetes mellitus and sarcopenia.
Methods
Electronic databases, including PubMed, EMBASE, Web of Science, and the Cochrane database, were searched from November to December 2021 for publications based on exercise intervention in patients with sarcopenia. After the selection of studies for this analysis, patients with diabetes mellitus were retrieved. Since dichotomous data including mean and standard deviation were reported, weighted mean difference (MD) with 95% confidence intervals (CI) were used to represent the data following analysis.
Results
A total of 431 participants with diabetes mellitus and sarcopenia were included in this meta-analysis. A statistical analysis was carried out on patients with diabetes mellitus who were assigned to the exercise intervention group. Our analysis showed that “sit-to-stand test” and “timed up and go” were significantly in favor of exercise intervention: MD −1.57, 95% confidence interval (CI) −2.26 to −0.87 (P = 0.0001) versus MD −0.61, 95% CI −1.21 to −0.01 (P = 0.05), respectively. Handgrip strength, walking speed and leg strength were also assessed. Another statistical analysis was carried out, this time on patients with diabetes mellitus and sarcopenia who were not assigned to an exercise intervention. The results showed no significant difference among sit-to-stand test, timed up and go, handgrip strength, and leg strength.
Conclusion
Exercise intervention significantly improved the time taken to stand up from a sitting position, and to “stand up and go” in patients with diabetes mellitus and sarcopenia. Therefore, exercise intervention should be considered a relevant therapy for such patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sarcopenia is defined as a progressive and generalized muscle disorder that involves accelerated loss of muscle mass and impaired function. |
It is believed to influence the ability to carry out daily activities, muscle strength, and physical capacity in the elderly. |
Studies have shown that sarcopenia has been implicated as both a cause and a consequence of diabetes mellitus. |
In this analysis, we aimed to systematically show the impact of exercise intervention as a therapy for patients with diabetes mellitus and sarcopenia. |
Exercise intervention significantly improved the time taken to stand up from a sitting position, and to “stand up and go” in patients with diabetes mellitus and sarcopenia. |
Therefore, exercise intervention should be considered a relevant therapy for such patients. |
Introduction
Sarcopenia is defined as a progressive and generalized muscle disorder that involves accelerated loss of muscle mass and impaired function that could result in serious adverse outcomes, including functional decline and physical incapability, falls and fractures, frailty, and mortality [1]. With advanced age, there is an inevitable decline in muscle mass, showing that sarcopenia usually develops with increased age [2]. Sarcopenia is believed to influence the ability to carry out daily activities [3], muscle strength, and physical capacity in the elderly [4]. It has been estimated that 5–13% of the elderly population aged between 60 and 70 years are affected by sarcopenia, and this number increases up to 11–50% among elderly people above 80 years of age [4]. Sarcopenia is one of the main reasons for loss of muscle mass. Several tests, including the short physical performance battery test, the timed-up-and-go test, and the chair-power climbing test could be used to assess muscle strength in patients with sarcopenia. Preventing or decreasing the intensity of sarcopenia could prevent further problems related to physical capacity [5]. Patients with diabetes mellitus are at greater risk of such physical incapability due to the impact of this chronic disease on their health.
Studies have shown that sarcopenia has been implicated as both a cause and a consequence of diabetes mellitus [6]. This chronic disease is characterized by insulin resistance, oxidative stress, increased advanced glycation end products, and a proinflammatory phenotype, which could result in macro- and microvascular complications and further interfere with normal cellular functioning and cause cell death, potentially leading to loss of skeletal muscle mass, strength, and function, resulting in sarcopenia [7]. Conversely, low muscle mass and function in sarcopenia could lead to weaker glucose disposal and reduced metabolic rate and physical activity, all of which might place elderly with sarcopenia at higher risk for developing diabetes mellitus [8].
Diabetes mellitus could worsen the condition, resulting in further impairment in daily activities of patients with sarcopenia. Unfortunately, treatment of sarcopenia could be challenging. Whether physical exercise could be a therapy for patients with diabetes mellitus and sarcopenia is not known. In this analysis, we aimed to systematically show the impact of exercise intervention as a therapy for patients with diabetes mellitus and sarcopenia.
Methods
Search Databases
Electronic databases, including PubMed, EMBASE, Web of Science and Cochrane database, were searched from November to December 2021 for publications based on exercise intervention in patients with sarcopenia. Reference lists of selective publications were also searched for relevant publications.
Search Strategies
Our search strategies included the following words or phrases:
-
Exercise and sarcopenia;
-
Exercise intervention and sarcopenia;
-
Exercise intervention, diabetes mellitus and sarcopenia;
-
Exercise intervention and frail adults;
-
Exercise and frail adults;
-
Exercise, frail adults and diabetes mellitus;
-
Physical exercise and sarcopenia;
-
Physical exercise and frail adults.
Inclusion and Exclusion Criteria
Studies were included if:
-
They were based on exercise intervention in patients with sarcopenia or frail patients [9];
-
They also included participants with diabetes mellitus;
-
They reported endpoints;
-
They were published in English.
Studies were excluded if:
-
They were systematic reviews, meta-analyses, or literature reviews;
-
They were case studies;
-
They did not include patients with diabetes mellitus;
-
They were duplicated studies that were repeatedly obtained through different search databases.
Outcomes and Exercise Interventions
The following endpoints were assessed:
-
(a)
Handgrip strength: defined as the maximum static force that a hand can squeeze;
-
(b)
Knee extension strength;
-
(c)
Walking speed;
-
(d)
Sit-to-stand test: defined as a way to assess an individual’s leg strength and endurance by having them stand up from a sitting position repeatedly over the course of 30 s;
-
(e)
Timed up and go: defined as a test where subjects are asked to rise from a standard armchair, walk to a marker 3 m away, turn, walk back, and sit down again. Timed up and go was reported on the basis of the total time taken to carry out this specific physical activity, and the lesser time taken was considered as a success;
-
(f)
Leg strength: defined as the ability of your legs to hold a contraction over time.
The outcomes reported in each original study are listed in Table 1.
Data Extraction and Quality Assessment
Six authors were involved in the data extraction process. The authors independently extracted data including the authors’ names, the publication year, the type of study, the total number of patients with diabetes mellitus and sarcopenia who were assigned to the exercise intervention group and the control group, and the mean and standard deviation of the endpoints at baseline and during follow-up, pre- and post-exercise. If any disagreement occurred, it was carefully discussed among all the authors and a consensus was reached.
Quality assessment of the randomized trials was calculated on the basis of the recommendations by the Cochrane collaboration [10], whereas quality assessment of the observational studies was calculated on the basis of the criteria recommended by the Newcastle Ottawa Scale (NOS) [11].
Statistical Analysis
Statistical analysis was carried out by the RevMan 5.4 software. Heterogeneity was assessed by the Q statistic test, whereas a P value less or equal to 0.05 was considered statistically significant. Heterogeneity was also assessed by the I2 statistic test. A lower I2 value was considered to represent low heterogeneity, whereas a higher I2 value was considered to represent higher heterogeneity. A fixed statistical effect was used in this analysis.
Mean and standard deviation were reported in the original studies. The data analysis was represented by weighted mean difference (MD) with 95% confidence intervals (CI). Publication bias was visually represented through funnel plots. In addition, sensitivity analysis was also carried out by an exclusion method, whereas each study was excluded one by one and a new analysis was carried out each time to compare with the main one.
Compliance with Ethical Guidelines
This analysis included data that were previously published. None of the authors carried out experiments on animals or human beings. Therefore, ethical or board review approval was not required for this analysis.
Results
Search Outcomes
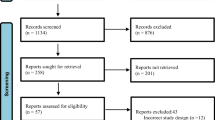
The Preferred Reporting Items in Systematic Reviews and Meta-analyses (PRISMA) guideline was followed [12]. Our search resulted in a total of 3128 publications. Over 3000 publications were eliminated after careful assessment of the titles and abstracts since they were not related to the scope of this analysis. Eighty four (84) full-text studies were assessed for eligibility. Following further eliminations based on the inclusion and exclusion criteria, only 14 studies [13,14,15,16,17,18,19,20,21,22,23,24,25,26] were finally included in this analysis. A flow diagram of the study selection is shown in Fig. 1.
General Features of the Studies
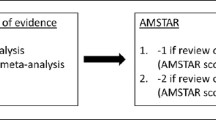
The general features of the studies are listed in Table 2. A total of 431 participants with diabetes mellitus and sarcopenia were included in this meta-analysis. The majority of the studies were randomized trials, whereas only three studies were observational studies. An assessment of the randomized controlled trials with the Cochrane risk assessment tool is demonstrated in Fig. 2. An assessment of the nonrandomized studies by the NOS was also carried out, and a grade B was allotted to the studies.
Baseline Features of the Studies
The baseline features are listed in Table 3. The mean age of the participants ranged from 65.3 to 84.1 years. Three studies included only female participants. The percentage of male participants ranged from 0.00% to 74.4%. The percentages of participants with comorbidities including hypertension and those who were smokers are listed in Table 3.
Main Results of this Analysis
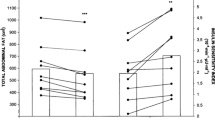
A statistical analysis was carried out on patients with diabetes mellitus who were assigned to the exercise intervention group. Our analysis showed that the sit-to-stand test and timed up and go were significantly in favor of exercise intervention with MD: −1.57, 95% CI −2.26 to −0.87 (P = 0.0001) and MD −0.61, 95% CI −1.21 to −0.01 (P = 0.05), respectively, as shown in Fig. 3. Handgrip strength (MD 0.56, 95% CI −1.28 to 2.40, P = 0.55), walking speed (MD 79.44, 95% CI 14.06–144.82, P = 0.02), and leg strength (MD 2.41, 95% CI 1.40–3.42, P = 0.00001) were also assessed as shown in Fig. 3.
Another statistical analysis was carried out, this time on patients with diabetes mellitus and sarcopenia who were not assigned to an exercise intervention. The results showed sit-to-stand test (MD 0.41, 95% CI −0.70 to 1.53, P = 0.47), timed up and go (MD −0.10, 95% CI −0.74 to 0.53, P = 0.75), handgrip strength (MD −0.16, 95% CI −3.00 to 2.68, P = 0.91), and leg strength (MD 0.01, 95% CI −1.05 to 1.06, P = 0.99) not to be significantly different, as shown in Fig. 4.
Publication bias was assessed through funnel plot as shown in Fig. 5.
Discussion
Our analysis showed that physical exercise significantly improved the time taken to stand up from a sitting position and the time taken to stand up and go. However, our results did not show any improvement in handgrip strength, walking speed, or leg strength in these patients with diabetes mellitus.
It has been shown that physical exercise might help to increase muscle strength in older adults, and improve brain volume, including gray and white matter regions, and help frail people to maintain stability while walking, thus preventing falls [4]. Similarly, in our analysis, physical exercise significantly improved the time taken to stand up from a sitting position and the time taken to stand up and go. This assessment was considered successful on the basis of the duration required to carry out those tasks pre- and post-exercise interventions in these patients with diabetes mellitus. Our analysis showed walking speed to significantly favor participants at baseline. However, only two studies were used to assess this outcome, and the level of standard deviation in both studies was high, which might have contributed to this significantly different result supporting patients at baseline.
Physical function usually declines with age, along with the ability to do physical activity. Muscle strength relates to the ability to move and lift objects, and it is measured by the power/force a person can exert and the amount of weight a person can bear or lift. Physical exercise has always contributed to good health. A recent study assessing the effect of home-based physical exercise on days at home in pre-frail and frail persons showed exercise intervention to prevent the deterioration of health-related quality of life [26]. Another randomized trial demonstrating the effect of resistance exercise on frailty and functional health in community-dwelling older adults showed a 3-month multifactorial intervention to have reduced frailty and improved functional health, and these effects persisted for at least 3 more months after the exercise intervention [25].
Moreover, a prospective observational study involving 97 elderly men and women aged 65 years and older showed that low-intensity body weight training with slow movement on motor function resulted in improvements in ambulatory function and lower limb muscle strength within a short time [18].
In a narrative review, based on the 14 studies focusing on the effects of physical activity interventions in frail and pre-frail community-dwelling people on frailty status, muscle strength, physical performance, and muscle mass, the authors concluded and supported the fact that physical exercise intervention has been effective at reducing frailty and increasing muscle strength and physical performance [27]. Resistance training has shown to be an effective way of increasing muscle mass and strength regardless of protein supplementation, leading to improvement in activities such as bench press and walking speed, as demonstrated by a recent study carried out by Maltais et al. based on elderly men with sarcopenia [28]. However, the percentage or number of participants with diabetes mellitus was not given. In another study, a randomized trial, focusing on the optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of 80 people with age-related muscle loss (sarcopenia), the authors showed that vibration training exercise improved and maintained knee extension performance during a 12-week follow-up time period, further supporting the benefits of exercise intervention in patients with sarcopenia [29].
Even though our results showed no improvement in handgrip strength, walking speed, or leg strength in these patients with diabetes mellitus, the very low number of participants, different criteria for defining sarcopenia, the inclusion of frail participants, a low number of studies assessing the respective outcomes, and missing information (duration of diabetes mellitus, glycemic control, racial status of the participants) may explain this result.
Limitations
Our study has several limitations. First of all, a limited number of original studies based on this idea were published. Therefore, retrieving participants with diabetes mellitus led to a low number of participants for this analysis. Secondly, although we included many relevant publications in this analysis, those with outcomes reported in percentages instead of seconds could not be included in statistical analysis. Another limitation was the fact that the follow-up time periods from baseline varied across studies. The duration of intervention was therefore not taken into consideration in this analysis. Moreover, we could not use data from all of the relevant studies because few studies reported outcomes that were unique and not reported in other studies for comparison. Hence, even though included in this study, we could not use their data for statistical analysis. In addition, among those studies included in this analysis, the diagnostic criteria for sarcopenia were unclear in some of the studies, which could have had an impact on the results of this analysis. We have also included both patients with sarcopenia and those with frailty in this analysis, which could also affect have affected the results of this analysis. Physical function impairment is common in both sarcopenia and frailty, and these two conditions are highly similar and have therefore not yet received a unique operational definition. Therefore, we included frail patients with diabetes mellitus and patients with diabetes mellitus and sarcopenia in this analysis. Another limitation could be that exercise frequency per week and intensity of exercise were not taken into consideration since these data were not regularly reported in the original studies. Also, as endpoints including skeletal muscle mass, skeletal muscle mass index, and lean body mass were not reported in most of the original studies, we could not assess these outcomes in our analysis. In addition, features such as duration of diabetes mellitus, glycosylated hemoglobin (HbA1c), patients with micro- and macrovascular complications, number of patients on insulin therapy, and number of patients on oral hypoglycemic drugs were not reported, and were therefore ignored in this analysis. Even though racial differences could have had an impact on the outcomes, for example, body mass index of the Asian population was lower compared with people from Western countries, we could not conduct this comparison in our analysis, demonstrating another limitation of this study.
Conclusion
Exercise intervention significantly improved the time taken to stand up from a sitting position, and to “stand up and go” in patients with diabetes mellitus and sarcopenia. Therefore, exercise intervention should be considered a relevant therapy for such patients. Further research with a larger population of participants with diabetes mellitus and sarcopenia is required to confirm our hypothesis.
References
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Aihie Sayer A, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel J-P, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Nishimura T, Imai A, Fujimoto M, Kurihara T, Kagawa K, Nagata T, Sanada K. Adverse effects of the coexistence of locomotive syndrome and sarcopenia on the walking ability and performance of activities of daily living in Japanese elderly females: a cross-sectional study. J Phys Ther Sci. 2020;32(3):227–32.
Von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cach Sarcop Muscle. 2010;1(2):129–33.
Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;20(132):42–9.
Scott D, de Courten B, Ebeling PR. Sarcopenia: a potential cause and consequence of type 2 diabetes in Australia’s ageing population? Med J Aust. 2016;205(7):329–33.
Wang T, Feng X, Zhou J, Gong H, Xia S, Qing Wei XuHu, Tao R, Li L, Qian F, Li Yu. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci Rep. 2016;6:38937.
Velázquez-Alva MC, Irigoyen-Camacho ME, Zepeda-Zepeda MA, Lazarevich I, Arrieta-Cruz I, D’Hyver C. Sarcopenia, nutritional status and type 2 diabetes mellitus: a cross-sectional study in a group of Mexican women residing in a nursing home. Nutr Diet. 2020;77(5):515–22.
Landi F, Calvani R, Cesari M, Tosato M, Martone AM, Bernabei R, Onder G, Marzetti E. Sarcopenia as the biological substrate of physical frailty. Clin Geriat Med. 2015;31(3):367–74.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group. Cochrane statistical methods group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343):d5928.
Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;6(7):e1000100.
Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, Holloszy JO. Effects of progressive resistance training on body composition in frail older adults: results of a randomized, controlled trial. J Gerontol Ser A Biol Sci Med Sci. 2005;60(11):1425–31.
Brovold T, Skelton DA, Bergland A. Older adults recently discharged from the hospital: effect of aerobic interval exercise on health-related quality of life, physical fitness, and physical activity. J Am Geriat Soc. 2013;61(9):1580–5.
Díaz EG, Ramírez JA, Fernández NH, Gallego CP, Hernández DD. Effect of strength exercise with elastic bands and aerobic exercise in the treatment of frailty of the elderly patient with type 2 diabetes mellitus. Endocrinología, Diabetes y Nutrición (English ed.). 2019;66(9):563-70.
Hsieh T-J, Shin-Chang Su, Chen C-W, Kang Y-W, Ming-Hsia Hu, Hsu L-L, Szu-Yun Wu, Chen L, Chang H-Y, Chuang S-Y, Pan W-H, Hsu C-C. Individualized home-based exercise and nutrition interventions improve frailty in older adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2019;16(1):119.
Brazo-Sayavera J, López-Torres O, Martos-Bermúdez Á, Rodriguez-Garcia L, González-Gross M, Guadalupe-Grau A. Effects of power training on physical activity, sitting time, disability, and quality of life in older patients with type 2 diabetes during the COVID-19 confinement. effects of power training on physical activity, sitting time, disability, and quality of life in older patients with type 2 diabetes during the COVID-19 confinement. J Phys Act Health. 2021;18(6):660–68.
Kanda K, Yoda T, Suzuki H, Okabe Y, Mori Y, Yamasaki K, Kitano H, Kanda A, Hirao T. Effects of low-intensity bodyweight training with slow movement on motor function in frail elderly patients: a prospective observational study. Environ Health Prev Med. 2018;23(1):4.
Kemmler W, von Stengel S, Engelke K, Häberle L, Mayhew JL, Kalender WA. Exercise, body composition, and functional ability: a randomized controlled trial. Am J Prevent Med. 2010;38(3):279–87.
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriat Soc. 2012;60(1):16–23.
Kim H, Kim M, Kojima N, Fujino K, Hosoi E, Kobayashi H, Somekawa S, Niki Y, Yamashiro Y, Yoshida H. Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc. 2016;17(11):1011–9.
Lai X, Bo L, Zhu H, Chen B, Zhao W, Hongdi D, Huo X. Effects of lower limb resistance exercise on muscle strength, physical fitness, and metabolism in pre-frail elderly patients: a randomized controlled trial. BMC Geriatr. 2021;21(1):447.
Giné-Garriga M, Guerra M, Pagès E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401–24.
Sanchis J, Sastre C, Ruescas A, Ruiz V, Valero E, Bonanad C, García-Blas S, Fernández-Cisnal A, González J, Miñana G, Núñez J. Randomized comparison of exercise intervention versus usual care in older adult patients with frailty after acute myocardial infarction. Am J Med. 2021;134(3):383-390.e2.
Seino S, Nishi M, Murayama H, Narita M, Yuri Yokoyama Yu, Taniguchi NY, Amano H, Kitamura A, Shinkai S. Effects of a multifactorial intervention comprising resistance exercise, nutritional and psychosocial programs on frailty and functional health in community-dwelling older adults: a randomized, controlled, cross-over trial. Geriatr Gerontol Int. 2017;17(11):2034–45.
Suikkanen SA, Soukkio PK, Aartolahti EM, Kautiainen H, Kääriä SM, Hupli MT, Sipilä S, Pitkälä KH, Kukkonen-Harjula KT. Effects of home-based physical exercise on days at home and cost-effectiveness in pre-frail and frail persons: randomized controlled trial. J Am Med Direct Assoc. 2021;22(4):773–9.
Haider S, Grabovac I, Dorner TE. Effects of physical activity interventions in frail and prefrail community-dwelling people on frailty status, muscle strength, physical performance and muscle mass—a narrative review. Wiener Klinische Wochenschrift. 2019;131(11):244–54.
Maltais ML, Ladouceur JP, Dionne IJ. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Condit Res. 2016;30(6):1680–7.
Wei N, Pang MY, Ng SS, Ng GY. Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (sarcopenia): a randomized controlled trial. Geriat Gerontol Int. 2017;17(10):1412–20.
Acknowledgements
Funding
No external funding or sponsorship was received for this study or publication of this article. This study was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
The authors Siyao Gao, Ling Yu, Guozhong Yi, Tong Li, Zhenyin Chen, and Jiawang Ding were responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the initial manuscript and revising it critically for important intellectual content. All the authors approved the final manuscript as it is.
Disclosures
The authors Siyao Gao, Ling Yu, Guozhong Yi, Tong Li, Zhenyin Chen, and Jiawang Ding declare that they have no competing interests.
Compliance with Ethics Guidelines
This meta-analysis is based on previously conducted studies and does not contain any new study with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article. References of the original papers involving the data source which have been used in this paper have been listed in the main text of this current manuscript. All data are publicly available in electronic databases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gao, S., Yu, L., Yi, G. et al. Exercise Intervention as a Therapy in Patients with Diabetes Mellitus and Sarcopenia: A Meta-Analysis. Diabetes Ther 13, 1311–1325 (2022). https://doi.org/10.1007/s13300-022-01275-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-022-01275-3