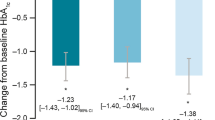Abstract
Introduction
This study investigated glycaemic control in individuals with type 1 (T1D) or type 2 diabetes (T2D) 6 months after initiating fast-acting insulin aspart (faster aspart) in a real-world setting.
Methods
This was a single-arm, observational study using extracted patient data from the IBM® Explorys® database (USA) for individuals with T1D or T2D initiating faster aspart (at least one prescription of faster aspart) in the study period 1 January 2018 to 27 October 2020. Clinical characteristics, including age, body mass index, and baseline HbA1c, were extracted, as well as recorded events of hypoglycaemia. The primary endpoint was the change in HbA1c from baseline to 6 months.
Results
A total of 787 individuals were included; 36.6% of these individuals had T1D and 63.4% had T2D (of whom 46.9% were new users of rapid-acting insulin when initiating faster aspart [T2D new users] and 53.1% were switching from another rapid-acting insulin to faster aspart [T2D switchers]). For individuals with T1D, T2D new users, or T2D switchers, estimated mean change in HbA1c from baseline to 6 months was − 0.20% (95% CI − 0.53, 0.14; p = 0.252), − 1.00% (95% CI − 1.34, − 0.67; p < 0.0001), and − 0.70% (95% CI − 1.06, − 0.35; p = 0.0001), respectively. In the baseline HbA1c > 8.5% subgroup, there was a significant estimated decrease in HbA1c from baseline to 6 months in individuals with T1D (− 1.2% [95% CI − 1.80, − 0.60]; p = 0.0001) or T2D (− 0.6% [95% CI − 0.92, − 0.35]; p < 0.0001). Event rates of hypoglycaemia after 12 months were 0.68, 0.38, and 0.59 events/year for individuals with T1D, T2D new users, and T2D switchers, respectively.
Conclusion
US IBM® Explorys® data demonstrated a clinically relevant reduction in HbA1c 6 months after initiating faster aspart treatment for individuals with T2D, but not T1D overall, although patients with baseline HbA1c > 8.5% had significant HbA1c reductions regardless of diabetes type.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Faster aspart is an ultra-fast-acting insulin analogue, with an improved pharmacological profile compared with conventional rapid-acting insulin analogues |
This study aimed to provide a real-world overview of the glycaemic control in a large cohort of people from US electronic records with type 1 diabetes, or people with type 2 diabetes who were new users of rapid-acting insulins, or switching to faster aspart |
The baseline characteristics of this patient population are reflective of a broad and clinically relevant study cohort |
A reduction in HbA1c was observed in individuals who were new users of rapid-acting insulins |
More real-world evidence studies are needed to observe the long-term outcomes of using faster aspart |
Introduction
Postprandial glucose (PPG) control is an important target for improving overall glycaemic control in people with diabetes [1], with guidelines stating that PPG control (in addition to fasting plasma glucose) is key for some patients to attain their HbA1c targets [2, 3]. However, good PPG control remains a challenge [4], in part due to the pharmacological limitations of widely used conventional rapid-acting insulin analogues. There is an unmet need for a more physiological, faster-acting mealtime insulin than the conventional alternatives for people with diabetes, to optimise PPG and overall glycaemic control.
Ultra-fast-acting insulin analogues, such as faster-acting insulin aspart (faster aspart), were developed to overcome these limitations [5]. Faster aspart, approved in 2017, is an improved formulation of insulin aspart (IAsp) for the treatment of children and adults with diabetes, and is available for use in multiple daily injections and continuous subcutaneous insulin infusion (insulin pumps) [6, 7]. Ultra-rapid lispro is another ultra-fast-acting insulin and was recently approved in 2020 [8]. A major advantage of the ultra-fast-acting insulins is the greater flexibility in the timing of injections compared with other currently available bolus insulins. Ultra-fast-acting insulins can be injected just before eating, or within 20 min after starting a meal [5], making it easier for patients to administer their mealtime bolus insulin.
Faster aspart offers an improved pharmacological profile compared with conventional IAsp in people with type 1 (T1D) or type 2 diabetes (T2D) [9, 10], and the faster and shorter action profile of faster aspart has been shown to translate into clinical benefits [11,12,13]. Thus, in the onset 1 and 8 clinical trials, which were treat-to-target, randomised phase 3 trials in individuals with T1D, change from baseline in HbA1c and 1-h PPG increment significantly favoured faster aspart over conventional IAsp [11,12,13]. In the onset 5 study, faster aspart was non-inferior to IAsp for the primary endpoint of change from baseline HbA1c 16 weeks after randomisation in individuals with T1D using continuous subcutaneous insulin infusion [7].
The efficacy and safety of faster aspart have also been investigated in individuals with T2D. The results of onset 2, a multicentre phase 3 trial in bolus-naïve individuals with T2D, demonstrated non-inferiority of faster aspart versus IAsp in reducing HbA1c [14]. Faster aspart improved 1-h PPG control versus IAsp with no differences observed in 2–4-h PPG levels [14]. Furthermore, the onset 9 trial investigated the use of faster aspart in individuals with T2D who switched their rapid-acting insulin to faster aspart following inadequate glycaemic control on a basal–bolus insulin regimen; faster aspart provided effective overall glycaemic control and superior PPG control versus IAsp [15].
While randomised clinical trials remain the gold standard for assessing efficacy and safety of new agents, their strict inclusion/exclusion criteria do not fully reflect the populations found in clinical practice [16]. For example, patients with hypoglycaemic unawareness are often excluded. Currently, there is limited real-world evidence on the use of faster aspart [17, 18], so real-world studies are needed to further assess and qualify the clinical benefits of faster aspart in a broad population of individuals who require insulin.
The aim of this study was to describe the clinical characteristics and to assess the change in glycaemic control of individuals with T1D or T2D upon initiation of faster aspart, based on data obtained from the US IBM® Explorys® database (International Business Machines Corporation, Armonk, NY, USA).
Methods
Study Design and Data Source
This was a single-arm, observational study based on data extracted from the US IBM® Explorys® database [19] (information available at https://www.ibm.com/downloads/cas/6VQK0DLL), which is a real-world collection of US electronic medical records (EMR) data updated once a week. Approximately 50 million people are followed in the US IBM® Explorys® database (ca. 18% of the US population) over an average of 3–4 years. The database contains outpatient, inpatient, and post-acute care data, covering information on patient demographics and disease characteristics, insurance, admissions and encounters, diagnoses, procedures and associated lab values, and surgeries. As this study was based on a historical observational cohort and only anonymous data were processed in this study, no informed consent was needed from the individuals involved, and approval from an institutional review board was not required.
Individuals
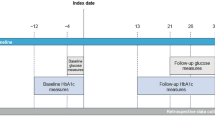
Included in the study were all individuals with T1D or T2D initiating faster aspart for the first time from 1 January 2018 to 27 October 2020. Individuals needed at least a 12-month database history prior to the index date, and baseline was defined as the date of first prescription of faster aspart. Prescriptions of faster aspart were identified on the basis of searches that included the brand name Fiasp® to differentiate from other insulin aspart-containing products (such as NovoLog®), and any of the following national drug codes: 01693201, 0169320111, 0169320190, 01693204, 0169320415, 0169320497, 0169320515, 0169320595 (Table S1 in the electronic supplementary material). T1D was defined as at least one T1D diagnosis code and no non-injectable glucose-lowering medication prescriptions, and T2D was defined as at least one T2D diagnosis code and at least one non-injectable glucose-lowering medication prescription (Table S1). Individuals with T2D initiating faster aspart were categorised as new users of rapid-acting insulin (no prescriptions of rapid-acting insulin [anatomical therapeutic chemical, or ATC, code A10AB] in the 12 months preceding the index date; T2D new users) or as one who switched rapid-acting insulin treatment to faster aspart (at least one prescription of rapid-acting insulin in the 12 months preceding the index date; T2D switchers). Low patient numbers were expected in the T1D new user group, and so all individuals with T1D were grouped together for the analysis and were not further stratified according to whether they were a new user of, or were switched to, faster aspart. Individuals were followed from the index date until the earliest event of 12 months after the index date, prescription of another rapid-acting insulin, or latest activity for the individual registered in the database. As this study was based on a historical observational cohort and only anonymous data were processed in this study, no informed consent was needed from the individuals.
Variables
All variables were defined based on data extracted from the database. Only records with dates within the range 01 January 1970 to 27 October 2020 were considered valid. Records of HbA1c, weight, and body mass index (BMI) were identified using pre-specified codes as defined in Table S1 in the electronic supplementary material. Latest values within 12 months prior to the index date were used as the baseline values. For HbA1c, all values until the end of follow-up were considered. In case of several records on the same date, the mean value was used. Only records with meaningful units and within biological realistic ranges (HbA1c, 2.5–20%; BMI, 10–150 kg/m2; weight, 1–600 kg) were considered (Table S1 in the electronic supplementary material). Glucose-lowering treatments before and at/after index date were identified as a record of prescription using ATC codes as defined in Table S1 in the electronic supplementary material. History of diabetes-related microvascular and macrovascular complications was identified by a record of an ICD9 or ICD10 diagnostic code (Table S1 in the electronic supplementary material) prior to index date. Likewise, history and incident event of hypoglycaemia were identified by a record of ICD9 or ICD10 (Table S1 in the electronic supplementary material). Age at index date was calculated on the basis of year of birth. The Charlson comorbidity index was extracted as recorded in the database and the latest value within 12 months prior to index date was used as the baseline value. Information on sex and race was also extracted; race was categorised as Caucasian, African American, other race, or multiple races.
Statistical Analysis
Baseline characteristics (demographics, BMI, Charlson comorbidity index, microvascular and macrovascular complications) of the study population and glucose-lowering treatment before and after initiation of faster aspart were reported by descriptive statistics according to diabetes type (T1D, T2D new user, T2D switcher): mean (standard deviation [SD]) for continuous variables and n (%) for categorical variables.
Hypoglycaemia events during the 12 months before and after initiating faster aspart were examined in individuals treated with insulin and/or sulfonylureas and reported descriptively as event rates per 100 person-years according to diabetes type (T1D, T2D new user, or T2D switcher).
HbA1c levels at 3, 6, 9, and 12 months after the index date were reported as descriptive statistics (number of individuals, % of mean, SD, median, 25th percentile, 75th percentile) and the proportions of individuals with different HbA1c levels were categorised using pre-specified ranges (< 7.0%, ≥ 7.0% to < 8.0%, ≥ 8.0% to < 9.0%, ≥ 9.0% to < 10.0%, ≥ 10%), both reported according to diabetes type. The primary outcome was the change in HbA1c from the index date to 6 months after the index date. To estimate this, mean HbA1c was further modelled over time by using a linear mixed model for repeated measurements (MMRM) (Proc Mixed in SAS Enterprise Guide 7.1).
Time was included as a categorical variable in the model (t = 0, 3, 6, 9, 12 months). An unstructured covariance matrix and Kenward–Roger’s approximation of the degrees of freedom were used. An interaction term between time and diabetes type was included in the model to examine HbA1c over time by diabetes type. Change in HbA1c from the index date to 6 months after the index date was estimated from this model using the LS MEANS statement and OBSMARGIN option. Further effect modifications were examined by including an interaction term between time and baseline HbA1c (≤ 8.5%, > 8.5%) and sodium-glucose co-transporter 2 inhibitor (SGLT2i)/glucagon-like peptide 1 receptor agonist (GLP-1 RA) use for T2D during the 12 months prior to the index date (yes/no).
Sensitivity analyses, including only individuals with evidence of a second prescription of faster aspart within 6 months after the index date, were conducted to test the robustness of the primary analysis of change in HbA1c.
Results
Patient Disposition and Baseline Characteristics
Patient disposition is summarised in Fig. 1. Overall, 904 individuals were identified in the database who had at least one prescription for faster aspart between 1 January 2018 and 27 October 2020, 852 (94.2%) of whom had a medical history at least 12 months prior to the index date. Of these, 65 individuals had other/unknown type of diabetes and were excluded, leaving 787 to be included in the current study. Overall, 36.6% (288/787) of individuals had T1D, while 63.4% (499/787) had T2D.
Of the individuals with T2D, 46.9% (234/499) started faster aspart treatment as their first rapid-acting insulin while 53.1% (265/499) switched to faster aspart from another rapid-acting insulin. Overall, discontinuation of faster aspart within the first 12 months occurred in 285 (36.2%) individuals: 97 individuals with T1D, 58 individuals with T2D who had started faster aspart, and 130 individuals with T2D who had switched to faster aspart from another rapid-acting insulin. The mean follow-up times for patients with T1D, T2D new users, and T2D switchers were 251, 278, and 251 days, respectively; the mean follow-up times for individuals who were exposed to faster aspart during the 12 months of follow-up were 180, 230, and 152 days, respectively.
Baseline characteristics are presented in Table 1. Individuals with T2D were older on average and had a higher mean BMI and mean baseline HbA1c than individuals with T1D. There was a higher proportion of individuals who had experienced previous hypoglycaemia and a history of diabetes-related microvascular/macrovascular complications in the T2D switcher subgroup versus the T2D new user subgroup.
HbA1c During the Follow-Up Period
The estimated mean HbA1c change from baseline to 6 months in individuals with T1D was − 0.20% (95% CI − 0.53, 0.14; p = 0.252) (Fig. 2). For individuals with T2D who were new users of or switchers to faster aspart, the estimated mean change in HbA1c from baseline to 6 months was − 1.00% (95% CI − 1.34, − 0.67; p < 0.0001) and − 0.70% (95% CI − 1.06, − 0.35; p = 0.0001), respectively (Fig. 2). Estimated mean change in HbA1c from baseline to 12 months for the T1D, T2D new user, and T2D switcher subgroups was − 0.15% (95% CI − 0.55, 0.25; p = 0.463), − 0.84% (95% CI − 1.24, − 0.43; p < 0.0001), and − 0.64% (95% CI − 1.12, − 0.16; p = 0.0095), respectively.
Estimated HbA1c over time during follow-up in the population stratified by diabetes type and T2D subgroup (new users versus switchers). Mean HbA1c was modelled over time by using a linear MMRM with time as a categorical variable. An unstructured covariance matrix and Kenward–Roger’s approximation of the degrees of freedom were used. Faster aspart, fast-acting insulin aspart; MMRM, mixed model for repeated measures; T1D, type 1 diabetes; T2D, type 2 diabetes
The primary outcome was further studied in subgroups of individuals stratified by baseline HbA1c ≤ 8.5% or > 8.5%, and stratifying by with/without documented use of SGLT2i/GLP-1 RA at or prior to baseline. In the baseline HbA1c > 8.5% subgroup, there was a significant estimated decrease in HbA1c 6 months post-index in individuals with T1D (− 1.2% [95% CI − 1.80, − 0.60]; p = 0.0001) or T2D (− 0.6% [95% CI − 0.92, − 0.35]; p < 0.0001) while no reduction was observed in the HbA1c ≤ 8.5% subgroups (Fig. 3). There was a significant estimated decrease in HbA1c 6 months post-index for both SGLT2i/GLP-1 RA users and non-users (SGLT2i/GLP-1 RA: − 0.8% [95% CI − 1.21, − 0.48]; p < 0.0001; no SLGT2i/GLP-1 RA: − 0.5% [95% CI − 0.78, − 0.32]; p < 0.0001) (Fig. S1 in the electronic supplementary material).
Estimated HbA1c during the 12-month follow-up period stratified by diabetes type and baseline HbA1c (≤ 8.5%, > 8.5%) at the index date. Mean HbA1c was modelled over time by using a linear MMRM with time as a categorical variable. An unstructured covariance matrix and Kenward–Roger’s approximation of the degrees of freedom were used. Further effect modifications were examined by including an interaction term between time and baseline HbA1c (≤ 8.5%, > 8.5%). MMRM, mixed model for repeated measures; T1D, type 1 diabetes; T2D, type 2 diabetes
The sensitivity analysis of the primary endpoint (individuals with at least two prescriptions of faster aspart within 6 months after the index date, as opposed to one) included 286 individuals (36.3% of the total population). The results of this sensitivity analysis showed similar trends to the base case described above (Fig. S2 in the electronic supplementary material).
The observed data for HbA1c and the proportion of individuals with HbA1c levels < 7.0%, ≥ 7.0% to < 8.0%, ≥ 8.0% to < 9.0%, ≥ 9.0% to < 10.0%, and ≥ 10% at baseline and during follow-up are presented in Table S2 in the electronic supplementary material.
Use of Insulin and Other Antidiabetic Medication
The percentage of individuals receiving different types of antidiabetic medication pre- and post-index date are shown in Table 2. The percentage of individuals who reported use of non-insulin antidiabetic medication was generally lower across all drug classes after the index date than before the index date.
Hypoglycaemia
In the 12 months prior to the index date, the event rates of hypoglycaemia for participants treated with insulin or sulfonylureas were 0.56, 0.16, and 0.58 events per person-year for individuals with T1D, T2D new users, and T2D switchers, respectively. In the 12 months after the index date, the event rates of hypoglycaemia were 0.68, 0.38, and 0.59 events per person-year for individuals with T1D, T2D new users, and T2D switchers, respectively.
Discussion
This observational study aimed to provide a real-world overview of the clinical characteristics and glycaemic control in individuals with T1D or T2D initiating faster aspart treatment. The majority of individuals in this cohort had T2D and, as might be expected, were on average older with a higher mean baseline HbA1c and BMI than individuals with T1D. Individuals with T2D switching to faster aspart were more likely to have a history of hypoglycaemia or microvascular/macrovascular complications than individuals who were new users of rapid-acting insulin treatment. Overall, HbA1c was statistically significantly reduced with faster aspart in individuals with T2D on the basis of differences between 6-month and baseline data, but this was not the case in individuals with T1D. However, HbA1c was significantly reduced in individuals with T1D or T2D with a baseline HbA1c level > 8.5%; this was not observed for those who already had better glycaemic control before switching to faster aspart (baseline HbA1c ≤ 8.5%). This is in agreement with previous studies, which found that patients with a baseline HbA1c of ≤ 8.5% had a lesser reduction in HbA1c in response to insulin treatment, compared with those with baseline HbA1c > 8.5% [20, 21]. Additional analyses showed that an improvement in HbA1c can be achieved with faster aspart, regardless of prior exposure to SGLT2i or GLP-1 RA. Sensitivity analyses in individuals who received at least two prescriptions of faster aspart (as opposed to at least one prescription) showed similar trends to the overall cohort. The sensitivity analyses intended to exclude individuals who might have discontinued treatment early or never picked up their prescription at the pharmacy. Therefore, the result may be a more accurate representation of people adhering to faster aspart treatment. Event rates of hypoglycaemia were similar before and after follow-up, except for individuals in the T2D new users subgroup, who experienced an increase in hypoglycaemic events as expected for individuals initiating insulin treatment.
The efficacy and safety of faster aspart have been investigated in randomised controlled trials both in people with T1D (onset 1 [11, 12] and onset 8 [13]) and in people with T2D (onset 2 [14] and onset 9 [15]). In onset 1, faster aspart was observed to be non-inferior compared with IAsp in HbA1c reduction when used as part of a basal–bolus insulin regimen in individuals with T1D [11, 13]. Faster aspart also demonstrated improved control of PPG excursions, without increased risk of overall severe or blood glucose-confirmed hypoglycaemia [11, 13]. In bolus insulin-naïve adults with T2D treated with basal insulin for at least 6 months, faster aspart improved 1-h PPG control versus IAsp with no differences observed in 2–4-h PPG levels [14]. In a different population of adults with T2D (onset 9; T2D duration at least 10 years), faster aspart provided effective overall glycaemic control with improved 1-h PPG control versus IAsp, both in combination with insulin degludec 100 units/mL [15]. Overall, the real-world findings in terms of glycaemic control reported in the current study, particularly for those individuals with elevated HbA1c at baseline, align with the results from randomised controlled trials [12,13,14,15].
This study features limitations inherent to using databases of medical records. As this was a one-arm study without a comparison group, the observed reduction in HbA1c may be attributable to factors other than initiation of faster aspart, such as increases in insulin dose, changes in other glucose-lowering medications during the study period, or the introduction of continuous glucose monitoring devices. Another limitation is the assumption that all individuals who were prescribed faster aspart both collected their medication at the pharmacy and took their medication; the database does not provide this level of information. Hypoglycaemia events were recorded in the database by a medical professional, so mild events not requiring a consultation with a medical professional may have gone unreported. It is also possible that some individuals were misclassified as T1D or T2D. The study benefits, however, from the requirement for a baseline history of 12 months before the index date, as this provides a good overview of medication use at baseline and increases the likelihood that the index date was indeed the first prescription of faster aspart.
This was a real-world study reflective of clinical practice and serves to generate further research hypotheses. The strengths of the current study include the large dataset from a database widely used for diabetes analyses [16]. The study population included both individuals with T1D and those with T2D who were new users or switching to faster aspart, and included individuals with diabetic complications and comorbidities. Thus, the findings are reflective of a broad and clinically relevant study cohort.
There are limited real-world studies with faster aspart [17, 18, 22]. One of these studies explored patient and physician perspectives regarding the use of faster aspart and highlighted the benefit of increased dosing flexibility with faster aspart compared with other rapid-acting mealtime insulins [17]. Danne et al. [18] reported a reduction in HbA1c (− 0.19%, p < 0.0001) for people with T1D who were using continuous glucose monitoring in a real-world setting; these results are comparable to the findings of the current study. One study in a Belgian real-world setting found that, when patients with T1D switched to faster aspart from traditional mealtime insulins, their time spent in range (blood glucose levels within 70–180 mg/dL; as assessed by continuous glucose monitoring) improved from 50.3% at baseline to 55.5% at 12 months (p < 0.001) [22]. Altogether, the results of the current study are aligned with previous real-world evidence studies and randomised controlled trials, showing that faster aspart is an effective treatment option for people with T2D or uncontrolled T1D (with a baseline HbA1c of > 8.5%), regardless of baseline characteristics, history of hypoglycaemic events, or previous medication use.
Conclusion
Using data from the US IBM® Explorys® database in individuals with a range of treatment backgrounds, we observed a clinically relevant reduction in HbA1c in individuals with T2D, but not T1D, 6 months after initiating faster aspart treatment. These results were also reflected after 12 months of faster aspart treatment. Although a statistically significant reduction in HbA1c was not demonstrated in individuals with T1D, this was likely to be due to the individuals who had an HbA1c level ≤ 8.5% at baseline. This is supported by the significant estimated decrease in HbA1c observed in individuals with T1D or T2D who were in the baseline HbA1c > 8.5% subgroup. These findings reflect a representative US study cohort and suggest that faster aspart is an effective treatment option for people with T2D or uncontrolled T1D.
References
Tibaldi J. Importance of postprandial glucose levels as a target for glycemic control in type 2 diabetes. South Med J. 2009;102:60–6.
International Diabetes Federation Guideline Development Group. Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract. 2014;103:256–68.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S111–24.
Evans M, Schumm-Draeger PM, Vora J, King AB. A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes Obes Metab. 2011;13:677–84.
Kruger DF, Novak LM. Role of ultrafast-acting insulin analogues in the management of diabetes. J Am Assoc Nurse Pract. 2019;31:537–48.
Novo Nordisk A/S. FDA FIASP prescribing information. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208751s000lbl.pdf. Accessed 01 Feb 2021.
Klonoff DC, Evans ML, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast-acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab. 2019;21:961–7.
Eli Lilly. Lilly Investors news releases: FDA approves Lyumjev™. 2020. https://investor.lilly.com/news-releases/news-release-details/fda-approves-lyumjevtm-insulin-lispro-aabc-injection-lillys-new. Accessed 04 Jan 2021.
Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56:551–9.
Pieber TR, Svehlikova E, Brunner M, Halberg IB, Due Thomsen KM, Haahr H. Fast-acting insulin aspart in people with type 2 diabetes: earlier onset and greater initial exposure and glucose-lowering effect compared with insulin aspart. Diabetes Obes Metab. 2019;21:2068–75.
Russell-Jones D, Bode BW, De Block C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care. 2017;40:943–50.
Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52-week, randomized, treat-to-target, phase 3 trial. Diabetes Obes Metab. 2018;2018:5.
Buse JB, Carlson AL, Komatsu M, et al. Fast-acting insulin aspart versus insulin aspart in the setting of insulin degludec-treated type 1 diabetes: efficacy and safety from a randomized double-blind trial. Diabetes Obes Metab. 2018;20:2885–93.
Bowering K, Case C, Harvey J, et al. Faster aspart versus insulin aspart as part of a basal-bolus regimen in inadequately controlled type 2 diabetes: the onset 2 trial. Diabetes Care. 2017;40:951–7.
Lane WS, Favaro E, Rathor N, et al. A randomized trial evaluating the efficacy and safety of fast-acting insulin aspart compared with insulin aspart, both in combination with insulin degludec with or without metformin, in adults with type 2 diabetes (onset 9). Diabetes Care. 2020;43:1710–6.
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35:1763–74.
Baru A, Amir S, Ekelund M, Montagnoli R, Da Rocha Fernandes JD. A survey of physician experience and treatment satisfaction using fast-acting insulin aspart in people with type 1 or type 2 diabetes. Postgrad Med. 2020;132:320–7.
Danne T, Schweitzer MA, Keuthage W, et al. Impact of fast-acting insulin aspart on glycemic control in patients with type 1 diabetes using intermittent-scanning continuous glucose monitoring within a real-world setting: the GoBolus study. Diabetes Technol Ther. 2020;23:201–12.
IBM Watson Health. Data sheet—IBM Explorys Electronic Health Record (EHR) Database. 2020. https://www.ibm.com/downloads/cas/6VQK0DLL. Accessed 01 Dec 2020.
Yu M, Yuan GY, Zhang B, Wu HY, Lv XF. Efficacy and safety of dulaglutide by baseline HbA1c in Chinese patients with type 2 diabetes: a post hoc analysis. Diabetes Ther. 2020;11:1147–59.
Kaneko S, Oura T, Matsui A, Shingaki T, Takeuchi M. Efficacy and safety of subgroup analysis stratified by baseline HbA1c in a Japanese phase 3 study of dulaglutide 0.75 mg compared with insulin glargine in patients with type 2 diabetes. Endocr J. 2017;64:1165–72.
Billion L, Charleer S, Verbraeken L, et al. Glucose control using fast-acting insulin aspart in a real-world setting: a 1-year, two-centre study in people with type 1 diabetes using continuous glucose monitoring. Diabetes Obesity Metabol. 2021. https://doi.org/10.1111/dom.14527.
Acknowledgements
Funding
This study and the journal’s Rapid Service Fee were funded by Novo Nordisk A/S.
Authorship Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship
LH and DM conducted the data analyses and had full access to the patient information received from the US IBM® Explorys® database; as such, they take responsibility for the integrity of the data and the accuracy of the data analysis. WL, MF, LH, DM, NR, and CDB all contributed to the interpretation of data and drafting of the manuscript.
Medical Writing Assistance
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, were provided by Carrie Fielden, MSc, Matthew Robinson, DPhil, and Helen Marshall, BA, of Ashfield MedComms, funded by Novo Nordisk A/S.
Disclosures
Certain data used in this study were supplied by International Business Machines Corporation. Any analysis, interpretation, or conclusion based on these data is solely that of the authors and not that of International Business Machines Corporation. Wendy Lane has served on advisory boards for Novo Nordisk, has received honoraria for serving on speakers’ bureaus for Novo Nordisk and Dexcom, and has received research grant support from Novo Nordisk. Mads Faurby, Lise Lotte N Husemoen, Dmitriy L. Markovich and Naveen Rathor are employees of Novo Nordisk A/S. Christophe De Block has received honoraria from AstraZeneca, Abbott Diagnostics, A. Menarini Diagnostics, Boehringer-Ingelheim, Roche Diagnostics, Eli Lilly, MSD, Novo Nordisk, Novartis and Sanofi; grants from AstraZeneca and Novo Nordisk; and lecture fees from Abbott Diagnostics, Eli Lilly, Novo Nordisk, Sanofi and Boehringer-Ingelheim.
Compliance with Ethics Guidelines
As this study was based on a historical observational cohort and only anonymous data were processed in this study, no informed consent was needed from the individuals involved, and approval from an institutional review board was not required.
Data Availability
The IBM Explorys Electronic Health Record (EHR) granted access to the database used in this study and, as such, the datasets generated during and/or analysed during the current study are not publicly available.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lane, W., Faurby, M., Husemoen, L.L.N. et al. Glycaemic Control in People with Diabetes Starting Treatment with Fast-Acting Insulin Aspart: a US Database Study. Diabetes Ther 12, 3067–3077 (2021). https://doi.org/10.1007/s13300-021-01165-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01165-0