Abstract
Introduction
To determine the association between mean glycated haemoglobin (HbA1c) or glycaemic variability and the development of diabetic retinopathy (DR) in people with diabetes.
Methods
An observational cohort study with people registered with a DR eye screening service between October 2012 and October 2017. Those who had no DR at the start of the study were followed for a maximum of 5 years. HbA1c measures were used to calculate HbA1c variability and mean HbA1c to assess any relationship between these and the risk of developing new onset DR.
Results
A total of 2511 individuals were followed up for up to 5 years. Of these, 542 (21.6%) developed DR. After adjustment, HbA1c variability was not significantly associated with the development of DR (p = 0.3435). However, the mean HbA1c was (p < 0.0001). Those with type 1 diabetes had an odds of 1.63 (95% CI 1.11–2.40) of a retinopathy diagnosis compared to those with type 2 diabetes.
Conclusions
We have shown that mean HbA1c is associated with an increased risk of developing diabetic retinopathy. However, after adjustment for sex, age, diabetes type and the mean, the HbA1c variability no longer remained significant. Our data suggest that optimizing long-term glycaemic control remains paramount.
Plain Language Summary
It has been known for a long time that if an individual’s diabetes is not as well controlled as it could be, then they are at increased risk of developing complications over many years. These complications include diabetes-related eye disease (retinopathy). For many years, the way of measuring how well someone’s diabetes was controlled was by measuring glycated haemoglobin (HbA1c) which looked at how much glucose was attached to a red cell. This study looked at whether variation in HbA1c over 5 years of follow-up was associated with greater risk of developing retinopathy, and if this relationship was stronger than just measuring HbA1c alone. Previous work has shown that people with greater variation in HbA1c are at increased risk of poor wound healing in those with diabetes-related foot ulcers. The present study looked at 2511 people who had no diabetes-related eye disease at the start of the study and who had been followed for 5 years. We found that variability in HbA1c was not associated with an increased risk of developing diabetes-related eye disease, but confirmed that the average HbA1c had the strongest relationship. Our data confirm that the focus of preventing diabetes-related eye disease should be on lowering HbA1c.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Glycaemic variability as measured by visit-to-visit changes in glycated haemoglobin (HbA1c) is becoming an increasingly important risk factor in the development in microvascular and macrovascular complications of diabetes. |
We have used a large single-centre database of people with diabetes having annual diabetic retinopathy screening followed over 5 years. |
What was learned from the study? |
We show that those who have no diabetic retinopathy at the start but who develop it over time have greater HbA1c variability than those who do not, but after adjustment, this variability is not a significant risk. However mean HbA1c has the strongest relationship to risk of development of retinopathy. |
These data suggest that focus should be on lowering mean HbA1c. |
Introduction
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes and is one of the leading causes of preventable blindness in the developed world among working-aged people [1, 2]. Depending on the part of the world, DR has an annual incidence of between 2.2% and 12.7% [3]. Blindness due to DR is estimated to account for just over 1 in 40 of all case of visual loss globally, and just under 1 in 50 cases of moderate or severe visual impairment [4]. The presence of DR is also an early maker for premature cardiovascular mortality [5]. Therefore, early identification of those people at highest risk of developing DR is an important part of enabling appropriate multiple risk factor intervention with the aim of reducing the incidence rates of end organ damage. Glycated haemoglobin (HbA1c) concentrations are currently used to monitor glycaemic control, and the association between elevated HbA1c measurements and the onset and progression of DR, as well as other microvascular and macrovascular complications, is widely recognised [6,7,8,9]. However, there remains a need to see if there are further predictors of risk in people with diabetes.
Recently, long-term glycaemic variability—the visit-to-visit variation in HbA1c measurements—has been proposed as a novel and alternative measure of glycaemic control [10]. HbA1c variation between visits can differ greatly even among individuals with similar mean HbA1c values. Glycaemic variability can be measured using several different methods [11,12,13]. Several studies have suggested an association between glycaemic variability and the development of diabetes complications, with a greater variability contributing to a greater risk [14,15,16,17,18]. Furthermore other data show that high glycaemic variability is associated with an increased risk of major adverse cardiovascular events and all-cause mortality [19, 20]. However, to our knowledge only a few studies have investigated the relationship between HbA1c variability and DR in people with diabetes [21,22,23,24,25,26,27]. Many of these were relatively small studies with conflicting results. As such the data on HbA1c variability and the risk of developing DR remains inconclusive.
The aim of this study was to determine whether glycaemic variability (as measured by visit-to-visit variation in HbA1c) was associated with the development of new-onset DR in individuals undergoing routine DR screening surveillance.
Methods
Study Design, Setting and Patients
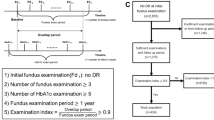
The project was registered with the audit department at our institution who deemed that ethical approval was not required because of the anonymised, retrospective nature of the data collection. This observational cohort study was conducted using data collected from our primary and secondary care records. The study population consisted of people registered with the DR eye screening service between October 2012 and October 2017. This covers the catchment population of our hospitals as well as those attending the specialist diabetes clinic, who have their eyes screened annually as part of their ‘annual review’. Eligible individuals were identified through the DR screening software, OptoMize® (EMIS Health, Leeds, UK). To be included in the ‘case’ group, subjects had to have no diagnosis of DR at baseline but have a documented diagnosis of DR by the end of the data collection period. The DR diagnosis could be of any stage and with or without the presence of maculopathy. To be included in the ‘control’ group, subjects had to have no diagnosis of DR at baseline, or at the end of the 5-year follow-up period. The diagnostic criteria for DR were those from the England and Wales National Screening Programme [28].
Additional inclusion criteria included a documented diagnosis of any type of diabetes mellitus, aetiology or treatment. The rationale for this was because whilst the majority of identified subjects would have their diabetes type recorded correctly, from previous experience we are aware that many people—including hospital inpatients (particularly those with type 2 diabetes on insulin)—may be incorrectly coded as having type 1 diabetes [29]. People were excluded if they had a pre-existing diagnosis of DR at baseline, or if they had fewer than three available HbA1c measurements during the 5-year follow-up period, or if all of their HbA1c measurements were during a period of less than 12 months. In addition, individuals in the ‘case’ group must have been screened for DR at least twice during the follow-up period—i.e. once to determine no retinopathy, then at least once more to determine onset of retinopathy.
Data Collection
For each individual, the baseline characteristics were recorded using hospital electronic records. Additional demographic data were collected where this was available. Data were collected over the 5-year follow-up period and every HbA1c measurement performed during this period was recorded.
Study Outcomes
The primary outcome was the development of new-onset DR during the follow-up period and assessing the impact that glycaemic variability had on the risk of developing DR. The presence of DR was assessed by accredited retinal screeners at the diabetic retinopathy eye screening clinic using seven-field stereoscopic fundal photography using Canon CR2 or CR2AF cameras (Canon Medical Systems Europe BV, Crawley UK). Confirmed DR was graded in accordance with the England and Wales National Screening Committee classification [28].
Statistical Analysis
Basic descriptive statistics were used to highlight the demographic variables for all subjects included in the study. The HbA1c variability for each individual was calculated as the standard deviation (SD) of all their recorded measurements across the 5-year follow-up period. Only those with a minimum of three recorded HbA1c measurements and which spanned a time period of 12 months or greater had their variability calculated. This was to ensure that there was sufficient data for a reliable calculation for each individual [17, 30]. Mean HbA1c was also calculated and subjects categorized into ‘low’ and ‘high’ mean groups. ‘Low’ mean HbA1c was defined as those individuals having a mean HbA1c ≤ 58 mmol/mol (7.5%) and ‘high’ mean HbA1c as > 58 mmol/mol (7.5%) [31]. The relationship between the mean HbA1c and HbA1c variability was analysed with variability classified as either ‘low’ or ‘high’ on the basis of the median. Further analysis of the effect of HbA1c variability was conducted by dividing the SD of HbA1c into quartiles to determine if there was a linear trend with increasing variability.
The glycaemic variability was also analysed as a categorical variable, categorized into quartiles to assess if there was a significant trend in retinopathy with increasing variability. Despite the large number of ways to calculate glycaemic variation, SD of HbA1c was chosen as it was the method most commonly used among studies in a recent systematic review [32]. It is simple to perform and therefore theoretically more plausible in a wider clinical setting. The hypothesis was that there is no difference between developing retinopathy versus those not developing retinopathy. An unadjusted analysis was performed using the chi-square test and the Wilcoxon rank sum test was performed to assess the impact of identified demographic and baseline variables.
To analyse the effect of HbA1c variability on the development of DR three measures of variability were analysed: (a) standard deviation of HbA1c values, (b) coefficient of variation and (c) range (difference between the maximum value and minimum value). The medians and their respective interquartile ranges were reported and tested between the two groups using a Wilcoxon rank sum test. A logistic regression was also performed to adjust for age and sex and to determine if there is any significant trend in increasing variability. In line with epidemiological data, diabetes was assumed to be type 2 diabetes for those in whom this was not recorded [33].
To adjust for potential confounding factors a logistic regression was performed to adjust for mean HbA1c, age, sex and estimated glomerular filtration rate (eGFR) at baseline. Unadjusted and adjusted odds ratios for the standard deviation categorized into quartiles have been reported.
For those that were diagnosed with retinopathy, a survival analysis was performed on time to diagnosis, to explore if HbA1c variability was also associated with time to diagnosis.
Results
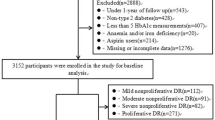
Figure 1 shows the CONSORT diagram for the subjects in the study. There were 3199 people registered in the database during the time period who had no retinopathy at baseline. After removal of those who did not meet the inclusion criteria, 2991 subjects remained, of whom 1998 (66.8%) had no retinopathy at the end of the 5-year study period and the remaining 993 (33.2%) had developed it.
To ensure that subjects in both groups had measurements recorded for similar durations, only subjects that had their last HbA1c measurement recorded in 2017 and 2016 were included in the analysis. For those subjects only the last 3 years’ measurements were included. Because of the skewed nature of the data, median numbers were used. This resulted in the ‘no retinopathy’ group having a median number of 6 visits (interquartile range 5–8), and the ‘retinopathy’ group having the same number of median of 6 visits (IQR 5–7). Because recordings had to be greater than 3 months apart this resulted in approximately two HbA1c measurements per year. However, 41 people (19 from the no retinopathy group, and 22 from the retinopathy group) had HbA1c measurements recorded within 3 months of a previously recorded value, and were therefore removed from the analysis (Fig. 1). This resulted in 2511 subjects included in the final analysis dataset, 1969 (78.4%) with no retinopathy diagnosis and 542 (21.6%) with a retinopathy diagnosis. Table 1 shows the descriptive statistics for this cohort.
Table 2 shows that when HbA1c variability was divided into quartiles of standard deviation, as HbA1c variability increased, the risk of developing retinopathy also increased. When the mean HbA1c was less than the overall median of 54 mmol/mol, the bottom SD quartile (SD < 3.2) (i.e. the lowest degree of HbA1c variability) the incidence of newly diagnosed retinopathy during the study was 14.9% compared to the top SD quartile (> 9.1) having an incidence of 26.9%. The same trend was seen for the mean HbA1c, with the lowest quartile (< 48 mmol/mol) having an incidence of 13.9%, compared to 32.6% when the mean was > 63 mmol/mol.
Supplementary Table S1 shows the correlation between the variability of HbA1c measurements and the mean of the measurements. There was a positive correlation of 0.55 between the mean HbA1c and the standard deviation of HbA1c measurements. Supplementary Table S1 also shows that the highest incidence of retinopathy was seen when HbA1c measurements were consistently over 63 mmol/mol and in the top three quartiles of variability.
Table 3 shows that when unadjusted, the HbA1c standard deviation had an odds of 1.027 (95% CI 1.01–1.04) of having a retinopathy diagnosis for every unit increase in the standard deviation (p = 0.0001). Similarly, the unadjusted HbA1c mean had an odds of 1.034 (95% CI 1.03–1.04) of having a retinopathy diagnosis for every unit increase in the mean.
After adjustment for sex, age, diabetes type and the mean, the standard deviation of HbA1c was not significant (p = 0.3435). However, the mean HbA1c itself was significant (p < 0.0001). These data support those in Supplementary Table S1 showing that the mean HbA1c had a stronger association than the variability with having a retinopathy diagnosis. Age was also significant (p = 0.0007), with an odds ratio of 1.01 (95% CI 1.01–1.02) of having a retinopathy diagnosis for every unit increase in age. Diabetes type was also a significant factor with type 1 diabetes having an odds of 1.63 (95% CI 1.11–2.40) of a retinopathy diagnosis compared to those with type 2 diabetes. This latter figure must be interpreted cautiously because for 90% of the subjects the diabetes type was not known, but was assumed to be type 2 diabetes because of the demographics or the catchment population of the screening service [34].
Figure S2 in the supplementary material shows a survival analysis for the 542 subjects that had a retinopathy diagnosis develop during the period of follow-up. This was done to explore if there was any difference in times to diagnosis between the mean HbA1c quartile bands. These data illustrate that the time taken to develop retinopathy was much the same in the bottom two quartiles, but are different from the two top HbA1c quartiles, which themselves are similar. For subjects in the top HbA1c quartile, the quartile estimate days to diagnosis was 1337 days (95% CI 1209–1438) and for the bottom quartile it was 1037 days (95% CI 729–1121).
Discussion
This observational study of 2511 subjects shows that the strongest risk for the development of diabetic retinopathy was a high mean HbA1c for the duration of the study. In addition, high glycaemic variability, as measured by the standard deviation of visit-to-visit HbA1c, over a period of at least 3 years was associated with a significantly increased risk of developing diabetic retinopathy; however, after adjustment for sex, age, diabetes type and the mean, HbA1c variability was no longer significant (p = 0.3435).
The association of chronic hyperglycaemia and the impact of achieving and maintaining optimal glycaemic control on the risk of developing DR was established by the Diabetes Control and Complications Trial [6]. The mechanisms by which chronic hyperglycaemia causes DR are complex, but involve regulation of blood pressure through the retina [35], ischemia and hyperosmotic damage, as well as sharing many of the other characteristics that are involved in the pathogenesis of diabetes-related microvascular disease [36, 37]. Until recently, however, little attention has been paid to the role of long-term variation in glycaemic control on the development of DR.
An analysis of the data from the Diabetes Control and Complications Trial using standard deviation (SD) as the measure of HbA1c variability showed a significant association between HbA1c variability and the risk of developing DR in type 1 diabetes [38]. Two additional studies then found no significant association when coefficient of variation (CV) was used as the measure of glycaemic variability [30, 39]. However, a 2015 meta-analysis found that DR appeared to be associated with HbA1c variability in people with type 1 diabetes, but not in type 2 diabetes [14].
Our data are in contrast to those from a small study of 220 people with type 1 diabetes which showed that the relationship between mean HbA1c and the risk of developing was strong (OR 2.82, 95% CI 1.45–5.5), but the relationship between the risk of developing DR and glycaemic variability as measured by CV of HbA1c was stronger, with an OR of 8.93 (95%CI 1.86–42.87) [31]. The increased risk for people with type 1 diabetes was also found in a large systematic review and meta-analysis of over 44,000 people with type 1 diabetes and 43,000 people with type 2 diabetes [14]. These authors showed that the risk associated with HbA1c variability was associated with an increased risk of retinopathy in those with type 1 diabetes (RR 2.11, 95% CI 1.54–2.89), but not for those with type 2 diabetes (RR 1.03 95% CI 0.69–1.53). Unlike our data, these results were independent of the mean HbA1c.
Our data are also different to those of Critchley et al. when considering the associations between mean HbA1c and outcomes in people with type 2 diabetes. Those authors showed that glycaemic variability was more associated with all-cause mortality than mean HbA1c, which was only important in those in the top and bottom HbA1c deciles [40]. We did not look at mortality, but showed that mean HbA1c was more important that HbA1c variability. This is similar to the conclusions reached by Cardoso et al. who suggested that mean HbA1c, rather than variability, was important in determining risk of developing retinopathy [23]. However, they found this relationship was strongest only in those with better controlled diabetes [23]. Thus the data are conflicting, with some studies showing no relationship between HbA1c variability and retinopathy in those with type 1 diabetes [30, 39], with other commentators suggesting there is one [38, 39]. Similarly, there are few data on people with type 2 diabetes, and some of those also suggest no relationship [21, 22]. However, recent data from a cohort of 1125 people from Park et al. have produced data similar to ours, suggesting that mean HbA1c and HbA1c standard deviation were associated with a lower risk of retinopathy and retinopathy progression [41]. Our data suggest that there is a relationship for those with type 2 diabetes—even though we have made the assumption that the vast majority of our cohort has type 2 diabetes. This assumption was made because of the demographics of type 1 and type 2 diabetes in the UK [33].
Thus, questions still remain as to the relative importance of long-term mean HbA1c or HbA1c variability. The former is the variable that has the longest and most data when considering risk of complications, but does not explain all of the risk [7, 38].
The time taken to diagnosis was longer in those in the highest HbA1c quartile. It may be that those individuals were least likely to seek help or attend routine scheduled appointments, thus had less optimal glycaemic control and also led to a delay in diagnosis. However, we accept that this is speculative.
We acknowledge that there are some limitations to our study. By the nature of the study it is observational and retrospective and so cause and effect is conjecture. We also excluded people with too few HbA1c measurements, or those who had them in a very short time frame. Furthermore, we did not have access to some of the residual or potential confounders that may influence the risk of developing retinopathy. In particular we did not know the duration of diabetes, because this was a predominantly primary care cohort and we did not have access to the primary care database. In addition, as a result of the nature of the study, we were unable to look at potential mechanistic contributions of variability of fasting plasma glucose, or concentrations of vascular endothelial growth factor, or insulin-like growth factor or lipid peroxides. Our cohort was predominantly Caucasian, potentially limiting the validity of our results in a more ethnically diverse population. In addition, we did not have access to co-morbidity status, e.g. the presence of hypertension, renal function or dyslipidaemia—or their treatments. Because of this, we were also unable to assess any potential relationship or confounding between type of treatment for diabetes, blood pressure or lipids, any treatment changes and subsequent impact on HbA1c variability. We were also unable to assess any potential legacy effect from previous tight glycaemic control (regardless of HbA1c variability). Song et al. showed that in their cohort of 604 people with type 2 diabetes, in addition to HbA1c variability, the 67 people who developed DR were more likely to have a longer duration of diabetes, higher body mass index, more likely to be insulin users and be hypertensive [25]. England has its own retinopathy grading system and thus we were not able to determine if our results may have been different using the Early Treatment Diabetic Retinopathy Scale [28]. In addition, low glycaemic variability may reflect a measure of retention of endogenous insulin secretion. Insulin is well recognized to have anti-inflammatory properties and may have offered a degree of protection to the retinal endothelium [42]. We also acknowledge that there are many different ways of measuring glycaemic variability and we have chosen the most frequently used, and easiest one to calculate [13].
There is also the limitation that we measured HbA1c variability at the same time as the outcome of interest and that this may have introduced a bias in terms of reverse causality, i.e. that the increased variability may be due to the closeness of the event, not the opposite. Other forms of bias may have been informative censoring (i.e. when reasons for loss to follow-up are confounded with the exposure), and to immortal time bias (i.e. that during the whole time period the exposure was being measured the individual is ‘immortal’, because, by definition, no endpoint can occur during this period) [43].
There is also the assumption that those with the highest HbA1c may have the highest variability, but that was not shown to be the case in another study looking at glycaemic variability and wound healing in diabetes-related foot ulcers [31]. It may also be argued that measurement of at least three HbA1c values during the 5-year period was not sufficient to show glucose variability and may underestimate it; however, we have previously used this methodology [31].
The strengths of our study are we have used a large unselected cohort of people with diabetes undergoing their routine annual eye screening appointment, with a long follow-up period. We have a single data capture system and only two senior retinopathy graders, ensuring little likelihood of missing or misdiagnosis. We also were able to exclude the potential impact of ‘early worsening’—i.e. those individuals who develop retinopathy with a rapid improvement in glycaemic control. This is because this condition usually develops in those who have a degree of retinopathy to start with—a group we excluded [44].
Conclusions
In summary, we have shown that in this large, single-centre cohort study, the strongest risk for the development of diabetic retinopathy was a high mean HbA1c. High glycaemic variability, as measured by unadjusted visit-to-visit change in HbA1c, was associated with an increased risk of developing diabetic retinopathy but the association no longer remained significant after adjustment.
References
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36.
Quartilho A, Simkiss P, Zekite A, et al. Leading causes of certifiable visual loss in England and Wales during the year ending 31 March 2013. Eye. 2016;30:602–7.
Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol. 2019;7:140–9.
Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643–9.
Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes. Diabetes Care. 2011;34:1238–44.
The Diabetes Control and Complications Trial Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86.
DCCT/EDIC research group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the Diabetes Control and Complications Trial. Diabetes. 1995;44:968–83.
United Kingdom Prospective Diabetes Study. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Hainsworth DP, Bebu I, Aiello LP, et al. Risk factors for retinopathy in type 1 diabetes: the DCCT/EDIC study. Diabetes Care. 2019;42:875–82.
Umpierrez GE, Kovatchev P. Glycemic variability: How to measure and its clinical implication for type 2 diabetes. Am J Med Sci. 2018;356:518–27.
Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–82.
Gomez AM, Munoz OM, Marin A, et al. Different indexes of glycemic variability as identifiers of patients with risk of hypoglycemia in type 2 diabetes mellitus. J Diabetes Sci Technol. 2018;12:1007–15.
Eslami S, Taherzadeh Z, Schultz MJ, Abu-Hanna A. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med. 2011;37:583–93.
Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015;38:2354–69.
Virk SA, Donaghue KC, Cho YH, et al. Association between HbA1c variability and risk of microvascular complications in adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2016;101:3257–63.
Dorajoo SR, Ng JS, Goh JH, et al. HbA1c variability in type 2 diabetes is associated with the occurrence of new-onset albuminuria within three years. Diabetes Res Clin Pract. 2017;128:32–9.
Luk AO, Ma RC, Lau ES, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013;29:384–90.
Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with type 2 diabetes. Diabet Med. 2016;33:1640–8.
Ghouse J, Skov MW, Kanters JK, et al. Visit-to-visit variability of hemoglobin A1c in people without diabetes and risk of major adverse cardiovascular events and all-cause mortality. Diabetes Care. 2019;42:134–41.
Kaze AD, Santhanam P, Erqou S, Ahima RS, Echouffo-Tcheugui JB. Long-term variability of glycemic markers and risk of all-cause mortality in type 2 diabetes: the look AHEAD study. BMJ Open Diabetes ResCare. 2020;8: e001753.
Penno G, Solini A, Bonora E, et al. HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes. Diabetes Care. 2013;36:2301–10.
Foo VH, Tan GS, Sabanayagam C, et al. HbA1c variability and diabetic retinopathy in Asian type 2 diabetes. Investig Ophthalmol Vis Sci. 2014;55:4412–4412.
Cardoso CR, Leite NC, Moram CB, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: the Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018;17:33.
Chang C-H, Chuang L-M. Glycated hemoglobin variability and retinopathy progression in type 1 diabetes: Is month-to-moth instability a better predictor? J Diabetes Investig. 2014;5:149–52.
Song K-H, Jeong J-S, Kim MK, et al. Discordance in risk factors for the progression of diabetic retinopathy and diabetic nephropathy in patients with type 2 diabetes mellitus. J Diabetes Investig. 2019;10:745–52.
Yang C-Y, Su P-F, Hung J-Y, Ou H-T, Kuo S. Comparative predictive ability of visit-to-visit HbA1c variability measures for microvascular disease risk in type 2 diabetes. Cardiovasc Diabetol. 2020;19:105.
Takao T, Suka M, Yanagisawa H, Matsuyama Y, Iwamoto Y. Predictive ability of visit-to-visit variability in HbA1c and systolic blood pressure for the development of microalbuminuria and retinopathy in people with type 2 diabetes. Diabetes Res Clin Pract. 2017;128:15–23.
Harding S, Greenwood R, Aldington S, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med. 2003;20:965–71.
Daultrey H, Gooday C, Dhatariya K. Increased length of inpatient stay and poor clinical coding: audit of patients with diabetes. J R Soc Med Short Rep. 2011;2:83.
Hermann JM, Hammes HP, Rami-Merhar B, et al. HbA1c variability as an independent risk factor for diabetic retinopathy in type 1 diabetes: a German/Austrian multicenter analysis on 35,891 patients. PLoS One. 2014;9: e91137.
Dhatariya KK, Li Ping Wah-Pun Sin E, Cheng JO, et al. The impact of glycaemic variability on wound healing in the diabetic foot—a retrospective study of new ulcers presenting to a specialist multidisciplinary foot clinic. Diabetes Res Clin Pract. 2018;135:23–9.
Nusca A, Tuccinardi D, Albano M, et al. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev. 2018;34: e3047.
National Diabetes Audit - Report 1 care processes and treatment targets 2018–19. Full report, 2020. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-1-care-processes-and-treatment-targets-2018-19-full-report. Accessed Aug 13, 2021.
Norfolk and Waveney STP diabetes strategy 2018–2023, 2018. https://www.knowledgeanglia.nhs.uk/LinkClick.aspx?fileticket=8Op2g2gsENY%3D&tabid=791&portalid=1&mid=953. Accessed Aug 13, 2021.
Kohner EM, Patel V, Rassam SM. Role of blood flow and impaired autoregulation in the pathogenesis of diabetic retinopathy. Diabetes. 1995;44:603–7.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.
Costantino S, Paneni F, Battista R, et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes. 2017;66:2472–82.
Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2008;31:2198–202.
Hietala K, Waden J, Forsblom C, et al. HbA1c variability is associated with an increased risk of retinopathy requiring laser treatment in type 1 diabetes. Diabetologia. 2013;56:737–45.
Critchley JA, Carey IM, Harris T, DeWilde S, Cook DG. Variability in glycated hemoglobin and risk of poor outcomes among people with type 2 diabetes in a large primary care cohort study. Diabetes Care. 2019;42:2237–46.
Park JY, Hwang JH, Kang MJ, et al. Effects of glycemic variability on the progression of diabetic retinopathy among patients with type 2 diabetes. Retina. 2020;41:1487–95.
Chaudhuri A, Umpierrez GE. Oxidative stress and inflammation in hyperglycemic crises and resolution with insulin: implications for the acute and chronic complications of hyperglycemia. J Diabetes Complicat. 2012;26:257–8.
Stevens SL, Wood S, Koshiaris CL, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354: i4098.
The Diabetes Control and Complications Trial Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications trial. Arch Ophthalmol. 1998;116:874–86.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
AH, AHa, RW and MJ collected the data, did the initial data analysis and wrote the initial draft of the manuscript. IN analysed the data. KD conceived the study and wrote the final draft of the manuscript. All authors saw and approved the final draft.
Disclosures
Ketan Dhatariya has nothing to disclose. Alexander Humberstone has nothing to disclose. Abul Hasnat has nothing to disclose. Rebecca Wright has nothing to disclose. Morgan Lujan has nothing to disclose. Ian Nunney has nothing to disclose.
Compliance with Ethics Guidelines
The project was registered with the audit department at our institution who deemed that ethical approval was not required because of the anonymised, retrospective nature of the data collection.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Dhatariya, K., Humberstone, A., Hasnat, A. et al. The Association Between Mean Glycated Haemoglobin or Glycaemic Variability and the Development of Retinopathy in People with Diabetes: A Retrospective Observational Cohort Study. Diabetes Ther 12, 2755–2766 (2021). https://doi.org/10.1007/s13300-021-01146-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01146-3