Abstract
Introduction
We investigated the safety of, and glucose control by, the insulin-only configuration of the iLet® bionic pancreas delivering fast-acting insulin aspart (faster aspart), using the same insulin-dosing algorithm but different time to maximal serum drug concentration (tmax) settings, in adults with type 1 diabetes.
Methods
We performed a single-center, single-blinded, crossover (two 7-day treatment periods) escalation trial over three sequential cohorts. Participants from each cohort were randomized to a default tmax setting (t65 [tmax = 65 min]) followed by a non-default tmax setting (t50 [tmax = 50 min; cohort 1], t40 [tmax = 40 min; cohort 2], t30 [tmax = 30 min; cohort 3]), or vice versa, all with faster aspart. Each cohort randomized eight new participants if escalation-stopping criteria were not met in the previous cohort.
Results
Overall, 24 participants were randomized into three cohorts. Two participants discontinued treatment, one due to reported ‘low blood glucose’ during the first treatment period of cohort 3 (t30). Mean time in low sensor glucose (< 54 mg/dl, primary endpoint) was < 1.0% for all tmax settings. Mean sensor glucose in cohorts 1 and 2 was significantly lower at non-default versus default tmax settings, with comparable insulin dosing. The mean time sensor glucose was in range (70–180 mg/dl) was > 70% for all cohorts, except the default tmax setting in cohort 1. No severe hypoglycemic episodes were reported. Furthermore, there were no clinically significant differences in adverse events between the groups.
Conclusion
There were no safety concerns with faster aspart in the iLet at non-default tmax settings. Improvements were observed in mean sensor glucose without increases in low sensor glucose at non-default tmax settings.
Trial Registration
ClinicalTrials.gov, NCT03816761.
Plain Language Summary
One way to give insulin is to use an insulin delivery system. The iLet® is a new type of insulin delivery system that works together with a continuous sugar monitoring tool (CGM). The CGM shows the blood sugar level in the body throughout the day. Based on this, the iLet automatically gives the insulin that is needed to control the blood sugar. Fast-acting insulin aspart (faster aspart) is a type of insulin that doctors can prescribe for use with insulin pens and insulin pumps. The researchers wanted to test the safety of faster aspart when given to people at different delivery settings in the iLet. Twenty-four men and women with type 1 diabetes from the USA took part. The different insulin delivery settings were the standard setting (tmax65 = 65 min) and new settings (tmax50 = 50 min; tmax40 = 40 min; tmax30 = 30 min). The shorter the tmax setting, the faster the insulin was assumed to be absorbed into the body by the iLet. People had good blood sugar control with faster aspart delivered using the iLet. The time with low blood sugar (i.e., < 54 mg/dl) was low for both the standard setting and the new settings. The average blood sugar was lower with the shorter, non-standard tmax settings. No people had serious side effects. No severe hypoglycemic episodes were reported. In this study, researchers found that it was safe to use faster aspart with the different settings in the iLet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Fast-acting insulin aspart (faster aspart) has a faster absorption profile than insulin aspart (IAsp) |
The iLet® bionic pancreas uses a dosing algorithm that is based on a bi-exponential pharmacokinetic model of the insulin |
We hypothesized that changing the time to maximal serum drug concentration (tmax) setting on the iLet bionic pancreas to optimally match the absorption profile of faster aspart would potentially deliver better glucose control than can be achieved using the iLet with faster aspart and the default tmax setting |
What was learned from the study? |
There were no safety concerns with faster aspart in the iLet at non-default tmax settings |
Improvements were observed in mean sensor glucose without increases in low sensor glucose at non-default tmax settings |
Overall, this study suggests that it may be valuable to optimize the tmax settings when using faster aspart in the iLet rather than using the default tmax settings |
Digital Features
This article is published with digital features, including a summary slide and plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14627199.
Introduction
There is an ongoing need to optimize glycemic control while reducing the risk of hypoglycemia in patients with type 1 diabetes, and advances have been made toward automated systems to assist with diabetes treatment [1]. Continuous subcutaneous insulin infusion (CSII) has been associated with favorable effects on glycemic control compared with multiple daily injections (MDI); however, the strength of evidence remains low and the observed differences in glycated hemoglobin (A1C) might not be considered clinically meaningful [2,3,4]. Furthermore, despite increased use of CSII and continuous glucose monitoring (CGM) systems, only a minority of patients with type 1 diabetes are achieving glycemic targets [5, 6], and hypoglycemia remains a concern [7].
A leap forward toward reaching treatment targets could be made with automated, closed-loop medical devices that use real-time data from CGM systems to inform mathematical algorithms that control insulin and/or glucagon delivery [8]. These systems can help reduce treatment burden, lower average glucose levels, and/or reduce hypoglycemia risk [8] by automatically adjusting the amount of insulin and/or glucagon infused in response to sensor glucose levels.
The iLet® bionic pancreas (iLet; Beta Bionics, Inc., Concord, MA, USA) is a purpose-built, standalone, wearable, medical device that requires only the user’s body weight for initialization. Based on user CGM readings, the iLet autonomously delivers insulin and/or glucagon to control blood glucose (BG) levels in response to the user’s real-time sensor glucose readings from a CGM [9]. The mathematical dosing algorithms used in the iLet for controlling sensor glucose levels do not require the user to count carbohydrates or to set or know their basal insulin rates, correction factors, or carbohydrate-to-insulin ratios [10]. The control algorithms used in the iLet consist of three insulin controllers running in parallel: (1) a basal insulin controller, which continually adapts to basal insulin needs; (2) a corrections controller, which continually adapts and provides control doses required above and beyond basal insulin; (3) a meal-announcement controller, which issues meal doses in response to meal announcements made by the user and continually adapts to the user’s prandial insulin needs [10]. No carbohydrate counting is required for meal announcements; rather, the user inputs via the iLet interface how the meal size compares with a typical meal for that time of day. When a meal announcement is made, the iLet gives 75% of the overall insulin for that meal type and chosen size, based on the dosing that was needed when similar meal announcements were made on previous days [9]. The iLet continues to adapt the meal dose on subsequent occasions when a meal announcement is made [9].
Previous randomized trials with the iLet in adults and children with type 1 diabetes have shown improved mean sensor glucose levels, increased time in range (70–180 mg/dl [3.9–10 mmol/l]) and reduced time in hypoglycemia (< 3.3 mmol/l) and hyperglycemia (> 10.0 mmol/l) versus the current standard of care [11, 12]. However, the absorption time and variable pharmacokinetics of conventional, rapid-acting insulin analogs may limit the extent to which glycemic control can be optimized. Automated insulin dosing systems might therefore benefit from an insulin with a faster absorption time and clearance rate than conventional, rapid-acting insulin analogs.
Fast-acting insulin aspart (faster aspart) is insulin aspart (IAsp) in a different formulation, as it contains two additional excipients: niacinamide and l-arginine. Faster aspart aims to mimic endogenous prandial insulin release more closely than IAsp [13]. l-arginine optimizes the stability of the formulation, while niacinamide increases early absorption of IAsp [13]. In a pooled analysis, pharmacokinetic (PK) and pharmacodynamic (PD) profiles for faster aspart were left-shifted versus those for IAsp, and onset of appearance in blood occurred earlier [14]. These pharmacological improvements were also observed after delivery by CSII [15]. In randomized clinical trials in adults using CSII, faster aspart was associated with significant improvements in postprandial glucose (PPG) control compared with IAsp [16, 17]. Faster aspart was also non-inferior to IAsp in terms of A1C [16]. The risk of overall severe or BG-confirmed hypoglycemia was similar for faster aspart and IAsp; however, a significantly higher rate of severe or BG-confirmed hypoglycemic episodes was reported within the first hour after the start of the meal with faster aspart versus IAsp [16]. Compatibility with CSII was similar for faster aspart and IAsp, demonstrated by an absence of microscopically-confirmed infusion-set occlusions for either insulin [18]. When using fully closed-loop insulin devices, both faster aspart and IAsp were well tolerated and performed similarly in achieving near-normal glucose concentrations outside postprandial periods in young adults with type 1 diabetes [19]. However, the closed-loop algorithm was not optimized for use with faster aspart in this study [19]. The iLet corrections controller continually estimates pending insulin action (related to ‘insulin-on-board’) by accounting for the time and magnitude of past insulin doses based on a bi-exponential PK model, which uses a single clinician-adjustable parameter: time to maximal serum drug concentration (tmax). The iLet default tmax setting is 65 min (t65), which has been used in clinical trials using insulin lispro [9]. In light of the faster absorption profile of faster aspart versus IAsp [14], we hypothesized that a shorter tmax (versus default tmax) would allow the iLet insulin dosing algorithms to more optimally match the PK profile of faster aspart and potentially deliver better glucose control than can be achieved using the iLet with faster aspart and the default tmax setting.
The study’s primary objective was to investigate the safety of selected tmax settings in the insulin-only configuration of the iLet using faster aspart in adults with type 1 diabetes in a short-term, outpatient clinical trial. A secondary objective was to investigate glucose control under non-default tmax settings compared with the default tmax setting for the iLet using faster aspart.
Methods
Trial Design
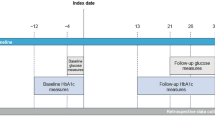
This was a single-center, sequential-cohort, randomized, single-blinded trial comprising three individual cohorts in adults with type 1 diabetes (NCT03816761). In each cohort, a non-default iLet tmax setting (t50 = 50 min, t40 = 40 min, and t30 = 30 min) was compared with the default iLet tmax setting (t65 = 65 min) in a two-period crossover design (Fig. 1). Participants were blinded to the order of the tmax settings in all study arms.
Trial design. *During the ‘in-patient’ period, participants stayed in a hotel close to the hospital. Participants were permitted to leave the hotel during the day; however, they informed the on-call study provider of their whereabouts. The ‘out-patient’ period was defined as the period after the participant had been discharged to home until the end of treatment. †Continuation to the next cohort only occurred if the following criteria did not occur in ≥ 2 subjects on the same tmax setting within a cohort: time in low sensor glucose > 2% of the total time on treatment; ≥ 1 treatment-emergent severe hypoglycemic episode. tmax, time to maximal serum drug concentration
Trial duration for each cohort consisted of a screening period, two 7-day treatment periods, and a 7-day follow-up period (Fig. 1). The 7-day treatment periods started with a 2-day ‘in-patient’ period, during which the participants stayed in a hotel close to the hospital. An on-call study provider stayed in the same hotel during this period. Participants were permitted to leave the hotel during the day; however, they informed the on-call study provider of their whereabouts. If the participant met safety criteria after this period of intensive safety surveillance, the participant was discharged home and continued on the iLet throughout the 5-day ‘out-patient’ period.
The non-default tmax setting that was compared with the default tmax setting was decreased when transitioning from one cohort to the next (from t50 for cohort 1 to t40 for cohort 2 and t30 for cohort 3). For each cohort, eight new participants were recruited and randomized. Continuation to the next cohort only occurred if the escalation-stopping criteria in the preceding cohort were not met. The criteria were: time in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) was > 2% of the total study duration while remaining on the tmax setting for that cohort or ≥ 1 episodes of treatment-emergent severe hypoglycemia occurred over the total study duration at a constant tmax setting. If any of these specified events occurred in two or more participants on the same tmax setting within a cohort, continuation to the next cohort with a shorter tmax setting would not occur.
Compliance with Ethics Guidelines
This trial was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments [20], International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice [21], International Organization for Standardization 14,155, and with US Food and Drug Administration 21 Code of Federal Regulations 312.120 [22]. The trial protocol, informed consent, and other relevant documents were reviewed and approved by local health authorities and an institutional review board (Partners Human Research Committee, ref. IRB00010760). Further details are provided in Table S1 in the Supplementary Material. All participants provided informed consent to participate in the study and publication of their clinical data for research purposes.
Study Population
In total, 38 participants were screened to achieve 24 participants randomly assigned to one of three treatment cohorts (randomization methodology included in the Supplementary Material). Participants were eligible if they were aged 18–75 years, diagnosed with type 1 diabetes at least 12 months prior to the day of screening, and had A1C ≥ 6.5– ≤ 9.0% (≥ 47– ≤ 75 mmol/mol) at screening. Eligible participants had to have been treated with CSII for at least 12 months prior to the day of screening. Additional inclusion criteria and full exclusion criteria are listed in the Supplementary Material.
Treatment Interventions
Throughout the study, the iLet was used in its insulin-only configuration (glucagon was not administered) in combination with faster aspart (100 units(U)/ml, 1.6 ml cartridge [NovoRapid® PumpCart®, Novo Nordisk, Bagsværd, Denmark]) and a real-time CGM sensor (Dexcom G5®, Dexcom, San Diego, CA, USA). Participants were randomized (1:1) to two different treatment sequences consisting of the default tmax setting (t65 = 65 min) followed by a non-default tmax setting (t50 = 50 min, t40 = 40 min, or t30 = 30 min), or vice versa, within their respective cohort: cohort 1, t65 versus t50; cohort 2, t65 versus t40; or cohort 3, t65 versus t30.
The iLet was initialized by site staff by entering the participant’s body weight. Participants were taught to use the meal announcement feature and were recommended to use it immediately before their main meals, although using the meal announcement was not a requirement. Details of the device training are included in the Supplementary Material. Site staff stopped the iLet at the end of a treatment period and reconfigured it to start again at the crossover visit.
After being initialized only with the user’s body weight, the mathematical control algorithms of the iLet autonomously determined insulin dosing and continually adapted to the participant’s insulin requirements based on real-time CGM sensor readings as well as automatically delivered meal doses of insulin in response to meal announcements. Therefore, no standardized scheme for insulin titration by study staff was needed. The glucose target for the iLet was set to 120 mg/dl (6.6 mmol/l). Participants were requested to maintain their diet and exercise habits as per their typical routines throughout the trial as much as possible.
Self-Measured Blood Glucose
Self-measured blood glucose (SMBG) values were obtained in the event of a hypoglycemic episode using a factory-calibrated BG meter to display plasma-equivalent glucose values. The participants used the SMBG values to calibrate the CGM sensor by entering the measured values into the study iPhone running the CGM app.
Real-time 24-h remote telemetric monitoring for hypoglycemia or persistent hyperglycemia was performed by site staff using a remote monitoring system, which generated an alarm if sensor glucose was < 50 mg/dl (2.8 mmol/l) for 15 min or > 300 mg/dl (16.6 mmol/l) for 90 min. When an alert was received, a site staff member called the participant and helped troubleshoot any issues, e.g., instructing participants to consume rapid-acting carbohydrates, measure their ketone levels, and/or change their infusion set.
Endpoints
The primary endpoint was the percentage of time in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) from initiation to end of treatment. Supportive secondary endpoints were also assessed from initiation to end of treatment. These included: percentage of time in sensor glucose range (70–180 mg/dl [3.9–10 mmol/l]); mean sensor glucose values; total daily insulin dose; number of treatment-emergent adverse events (TEAEs) and infusion-site reactions; number of severe hypoglycemic episodes; number of self-manageable treatment-emergent hypoglycemic episodes requiring oral carbohydrate intervention per day; and the number of treatment-emergent hypoglycemic episodes (overall, daytime, and nocturnal).
Hypoglycemic episodes were classified according to Novo Nordisk and American Diabetes Association (ADA) classifications of hypoglycemia [23]. Severe hypoglycemia was defined as an event requiring assistance of another person to actively administer carbohydrates, glucagon or take other corrective actions [23]. BG-confirmed hypoglycemia was defined as an episode confirmed by plasma glucose < 3.1 mmol/l (56 mg/dl), with or without symptoms consistent with hypoglycemia.
Additional Derivations
The coefficient of variation of the available sensor glucose values was calculated to assess sensor glucose profile variation.
Statistical Methods
No formal sample size calculations were made for the safety endpoints. Eight participants per cohort (24 participants in total) was deemed adequate to give an assessment of the primary objective.
The full analysis set (FAS) included all randomized participants receiving treatment. Participants in the FAS contributed to the evaluation ‘as treated’. The safety analysis set (SAS) included all participants receiving treatment. Participants in the SAS contributed to the evaluation ‘as treated’.
Assessment of the primary endpoint was based on descriptive summaries presenting mean, median, minimum, and maximum values by cohort and treatment. In a supplementary analysis of the primary endpoint, the default tmax setting was compared with each of the non-default tmax settings separately by cohort for time spent in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) using a linear mixed-effect model, with treatment and period as fixed effects, and participant as random effect.
Percentage of time in sensor glucose range (70–180 mg/dl [3.9–10 mmol/l]) and mean sensor glucose level were presented using descriptive statistics made by cohort and treatment and analyzed using a model similar to the supplementary analysis for the primary endpoint. Total daily insulin dose and self-manageable (able to self-treat) treatment-emergent hypoglycemic episodes requiring oral carbohydrate intervention were presented using descriptive statistics made by cohort and treatment. TEAEs, treatment-emergent infusion-site reactions, and treatment-emergent hypoglycemic episodes were summarized by cohort and treatment.
Results
Trial Participants
Of 38 screened participants, 24 entered into the trial and were allocated to 3 cohorts, each comprising 8 participants. All 24 participants were exposed to trial product (hence the FAS and SAS were identical), of whom 22 participants completed both treatment periods in their respective cohorts (Fig. S1 in the Supplementary Material). One participant in cohort 2 discontinued trial treatment after 4.5 days’ exposure in the second treatment period because the ‘participant moved out of town’. Another participant, in cohort 3, withdrew after 5.5 days of treatment exposure in the first treatment period (on the t30 setting) due to ‘low blood glucose’ as perceived by the participant, i.e., the participant was dissatisfied with their blood glucose control when using the iLet with the t30 setting in general.
Participant demographics and baseline characteristics are presented in Table S2 in the Supplementary Material. The observed mean body mass index (BMI) for cohorts 1 and 3 were similar (28.3 and 28.8 kg/m2, respectively) and greater than for cohort 2 (24.1 kg/m2). Duration of diabetes (16.6–33.1 years in all three cohorts) and age (36.3–47.1 years in all three cohorts) were lower in cohort 2 than cohorts 1 and 3 (Table S2 in the Supplementary Material).
Most participants were administering insulin lispro at screening (cohort 1, 75.0%; cohort 2, 87.5%; cohort 3, 100.0%); the remainder were administering insulin aspart.
The number of meal announcements made by participants according to the size of the meal are presented in Table S3 in the Supplementary Material.
Time of Treatment Period
The sensor glucose measurements from the CGM sensor for all three cohorts accounted for 84.0–88.9% of the total treatment time.
Time in Low Sensor Glucose (< 54 mg/dl [3.0 mmol/l])—Primary Endpoint
The observed mean time in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) was < 1.0% for all treatment arms (Fig. 2a–c; Table 1). Time in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) was 0.98% for t50 and 0.89% for t65 in cohort 1, 0.69% for t40 and 0.50% for t65 in cohort 2, and 0.61% for t30 and 0.37% for t65 in cohort 3.
Time spent in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) for a cohort 1, b cohort 2, and c cohort 3, and mean sensor glucose for d cohort 1, e cohort 2, and f cohort 3. Time spent in low sensor glucose was calculated as the percentage of available sensor glucose values below the threshold. Each blue line represents the sensor glucose profile for an individual participant in their respective cohort; different shades indicate different tmax settings. Yellow lines indicate mean over participants in a cohort. The blue dot represents the participant that discontinued treatment during the first treatment period, i.e., the participant has no assessment on the default (t65) setting. tmax, time to maximal serum drug concentration
The supplementary analysis of the primary endpoint, in which the within-participant treatment differences were investigated, did not show significant differences between arms in any of the cohorts (Table S4 in the Supplementary Material). Derivations evaluating time in low sensor glucose using cut-offs of < 60 mg/dl (3.3 mmol/l) and < 70 mg/dl (3.9 mmol/l) showed more time below these thresholds, as expected, but were consistent with the pattern observed for the < 54 mg/dl [3.0 mmol/l]) threshold (Table S5 in the Supplementary Material).
Mean Sensor Glucose Levels
Overall, mean sensor glucose was statistically significantly lower in cohort 1 for t50 (150.3 mg/dl [8.34 mmol/l]) versus t65 (157.7 mg/dl [8.75 mmol/l]); estimated treatment difference (ETD): − 7.36 mg/dl (− 12.31; − 2.41)95% CI, (− 0.41 mmol/l [− 0.68; − 0.13]95% CI), p = 0.011 (Fig. 2d, Table 1). In cohort 2, mean sensor glucose was statistically significantly lower for t40 (150.1 mg/dl [8.33 mmol/l]) versus t65 (157.6 mg/dl [8.75 mmol/l]); ETD: − 7.50 mg/dl (− 12.94; − 2.06)95% CI, (− 0.42 mmol/l [− 0.72; − 0.11]95% CI), p = 0.015 (Fig. 2e; Table 1). In cohort 3, mean sensor glucose was 144.1 mg/dl (8.00 mmol/l) for t30 and 152.5 mg/dl (8.46 mmol/l) for t65; ETD: − 7.73 mg/dl (− 16.84; 1.38)95% CI, (− 0.43 mmol/l [− 0.93; 0.08]95% CI), p = 0.082 (Fig. 2f; Table 1).
Time in Sensor Glucose Range (70–180 mg/dl [3.9–10 mmol/l])
Mean time in range was > 70% for all settings and cohorts except for the default tmax setting in cohort 1. Mean time in range was 70.9% for t50 and 66.8% for t65 in cohort 1, 73.9% for t40 and 70.4% for t65 in cohort 2, and 78.3% for t30 and 73.8% for t65 in cohort 3. ETD was 4.08% (− 3.36; 11.53)95% CI for cohort 1, 3.49% (− 2.05; 9.03)95% CI for cohort 2, and 4.49% (− 3.78; 12.76)95% CI for cohort 3 (Table 1).
Time in High Sensor Glucose (> 180 mg/dl [10 mmol/l])
Mean time spent in high sensor glucose across all cohorts was 18.75–29.33% (Table 1).
Additional Derivations
The coefficient of variation of the available sensor glucose values was calculated to assess the variation in the sensor glucose profiles. The coefficient of variation across all cohorts was 33.5–38.9% (Table 1).
Dosing
The total daily insulin dose was comparable across each cohort: 0.60 U/kg for t50 and 0.63 U/kg for t65 in cohort 1, 0.65 U/kg for t40 and 0.67 U/kg for t65 in cohort 2, and 0.63 U/kg for t30 and 0.64 U/kg for t65 in cohort 3.
TEAEs
Overall, there were no clinically relevant differences in TEAEs when using the non-default tmax settings versus the default tmax settings (Table S6 in the Supplementary Material). Seven TEAEs were reported by five participants across all tmax settings; none were considered serious or severe. There were no deaths, and none of the TEAEs led to withdrawal from the trial or permanent discontinuation of trial product.
Two TEAEs were reported as infusion-site reactions in participants on the t65 setting: one in cohort 3 with the preferred term ‘infusion-site reaction’ and the other in cohort 1 with the preferred term ‘hyperglycemia’ judged by the investigator as an infusion-site reaction. No lipodystrophy or allergic reactions were reported.
Hypoglycemia
The frequency and rate of hypoglycemic episodes are presented in Table 2. No severe hypoglycemic episodes were reported. The rate of BG-confirmed hypoglycemia was 99.20 episodes per year of exposure (PYE) for t50 and 105.40 episodes PYE for t65 in cohort 1, 92.01 episodes PYE for t40 and 48.43 episodes PYE for t65 in cohort 2, and 82.53 episodes PYE for t30 and 53.01 episodes PYE for t65 in cohort 3 (Table 2). Rates of daytime BG-confirmed hypoglycemia were 30.29–92.23 episodes PYE, and rates of nocturnal BG-confirmed hypoglycemia were 6.88–33.07 episodes PYE (Table 2).
The mean number of self-manageable hypoglycemic episodes per day requiring carbohydrate intervention was 1.01 episodes/day for t50 and 0.87 episodes/day for t65 in cohort 1, 1.06 episodes/day for t40 and 0.68 episodes/day for t65 in cohort 2, and 0.86 episodes/day for t30 and 0.51 episodes/day for t65 in cohort 3.
Safety Assessments
The number of infusion-set and PumpCart® changes was 24–38 across all cohorts (Table S7 in the Supplementary Material). Of these, 2–9 were non-routine changes (Table S7 in the Supplementary Material).
Discussion
This was the first randomized trial to investigate the use of faster aspart using the iLet under different tmax algorithm settings in adults with type 1 diabetes. Improvements in glucose control (reductions in mean sensor glucose and increases in time in the target range of 70–180 mg/dl [3.3–10 mmol/l] without clinically significant increases in hypoglycemia) were observed with faster aspart at non-default tmax settings (t50, t40, and t30) compared with the default tmax setting (t65). Importantly, no severe hypoglycemic episodes were reported, and there were no clinically significant differences in adverse events between groups. Based on a small sample of adults with type 1 diabetes, the results suggest that, in the insulin-only configuration of the iLet with faster aspart, using non-default values can improve glucose control relative to what is observed with the default tmax value, without compromising safety.
Mean time in low sensor glucose (< 54 mg/dl [3.0 mmol/l], primary endpoint) was < 1.0% for all of the studied tmax settings. The mean time in sensor glucose range (70–180 mg/dl [3.3–10 mmol/l]) was > 70%, except for the default tmax setting in cohort 1. These results broadly align with the ‘time in range’ targets recommended in international guidelines of spending < 1.0% of the time below 54 mg/dl [3.0 mmol/l] and > 70% of the time in range (70–180 mg/ml [3.9–10.0 mmol/l]). The results of the present study for average time in range for the three cohorts at the t65 using faster aspart was 67–74%. The average time in range for the non-default tmax values increased from t50 to t40 to t30, suggesting a greater time in range may be associated with shorter tmax values.
A similar trend was observed for mean sensor glucose levels, which were slightly higher for each of the three cohorts at the t65 setting compared with the non-default tmax values (t50, t40, and t30). This trend suggests a lower mean sensor glucose level may be associated with shorter tmax values. For each cohort, the total daily insulin dose was comparable at non-default versus default tmax settings. Taken together, these results suggest non-default tmax settings can be used in the iLet with faster aspart to improve glycemic control without compromising its safety profile.
There are several strengths to this study, including use of the iLet as the first automated insulin delivery system in which the tmax setting can be readily altered using the device’s graphical user interface. Furthermore, as the system only requires the user’s body weight for initialization, the burden on the patients and effect on daily activities are minimal. Faster aspart has been previously investigated in fully closed-loop systems [19, 24,25,26]; however, in these studies, the insulin dosing algorithm of the device was not optimized with the PK profile of faster aspart. Another strength of the current study is the crossover study design, which allowed the evaluation of within-participant tmax settings, thereby reducing the impact of inter-participant variability.
However, our study was not without limitations. First, carbohydrate intake to prevent hypoglycemia was not captured and may have confounded the hypoglycemia results. The remote monitoring may have reduced the duration and/or severity of hypoglycemic and hyperglycemic episodes compared with what might otherwise have occurred. Furthermore, the exclusion criteria resulted in a more homogeneous patient population than the population at large. Although the crossover design allowed the evaluation of within-participant tmax settings, the use of a new cohort of eight participants at each tmax setting limited our ability to make comparisons across the three non-default tmax settings. International guidelines recommend that 70% of CGM data from 14 days should be available for evaluation of the quality of glycemic control [27]. In this study ~ 84–89% of the total time of treatment was accounted for by CGM measurements (> 70%), the average total time of each study arm was 6–7 days.
Conclusions
We conducted a randomized, three-cohort, crossover trial in participants with type 1 diabetes to investigate each of the three non-default tmax settings (tmax = 50, 40, or 30 min) compared with the default tmax setting (tmax = 65 min) in the insulin-only configuration of the iLet using faster aspart. There were no severe hypoglycemic episodes, and no safety issues were identified. Based on the mean percentage of time spent in low sensor glucose (< 54 mg/dl [3.0 mmol/l]) being < 1% across all three cohorts, we concluded that safety of the glucose control was acceptable for the default and all non-default tmax settings. Additionally, mean sensor glucose for two of the three non-default tmax settings was statistically significantly lower than for the default tmax setting, with comparable total daily insulin doses. Overall, this study suggests that it may be valuable to optimize the tmax setting when using faster aspart in the iLet rather than using the default tmax value.
References
Nimri R, Nir J, Phillip M. Insulin pump therapy. Am J Ther. 2020;27:e30–41.
Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336–47.
Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010. https://doi.org/10.1002/14651858.CD005103.pub2.
REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ. 2017;356:j1285.
Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21:66–72.
van den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42:2050–6.
Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019;42:2220–7.
Pathak V, Pathak NM, O’Neill CL, Guduric-Fuchs J, Medina RJ. Therapies for type 1 diabetes: current scenario and future perspectives. Clin Med Insights Endocrinol Diabetes. 2019;12:1179551419844521.
El-Khatib FH, Russell SJ, Magyar KL, et al. Autonomous and continuous adaptation of a bihormonal bionic pancreas in adults and adolescents with type 1 diabetes. J Clin Endocrinol Metab. 2014;99:1701–11.
El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2:27ra.
El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389:369–80.
Russell SJ, Hillard MA, Balliro C, et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4:233–43.
Kildegaard J, Buckley ST, Nielsen RH, et al. Elucidating the mechanism of absorption of fast-acting insulin aspart: the role of niacinamide. Pharm Res. 2019;36:49.
Heise T, Pieber TR, Danne T, Erichsen L, Haahr H. A pooled analysis of clinical pharmacology trials investigating the pharmacokinetic and pharmacodynamic characteristics of fast-acting insulin aspart in adults with type 1 diabetes. Clin Pharmacokinet. 2017;56:551–9.
Heise T, Zijlstra E, Nosek L, Rikte T, Haahr H. Pharmacological properties of faster-acting insulin aspart vs insulin aspart in patients with type 1 diabetes receiving continuous subcutaneous insulin infusion: a randomized, double-blind, crossover trial. Diabetes Obes Metab. 2017;19:208–15.
Klonoff DC, Evans ML, Lane W, et al. A randomized, multicentre trial evaluating the efficacy and safety of fast-acting insulin aspart in continuous subcutaneous insulin infusion in adults with type 1 diabetes (onset 5). Diabetes Obes Metab. 2019;21:961–7.
Bode BW, Johnson JA, Hyveled L, Tamer SC, Demissie M. Improved postprandial glycemic control with faster-acting insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2017;19:25–33.
Zijlstra E, Demissie M, Graungaard T, Heise T, Nosek L, Bode B. Investigation of pump compatibility of fast-acting insulin aspart in subjects with type 1 diabetes. J Diabetes Sci Technol. 2018;12:145–51.
Dovc K, Piona C, Yesiltepe Mutlu G, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: a double-blind randomized crossover trial. Diabetes Care. 2020;43:29–36.
World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4.
The International Council for Harmonisation (ICH). ICH Harmonised Tripartite Guideline. Guideline for good clinical practice E6(R2), current step 4 version. 2016. https://www.ich.org/. Accessed Feb 01, 2021.
US Food and Drug Administration. Electronic code of federal regulations. 2021. https://www.ecfr.gov/cgi-bin/ECFR?page=browse. Accessed Feb, 01 2021.
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–95.
Hsu L, Buckingham B, Basina M, et al. Fast-acting insulin aspart use with the minimed(TM) 670G system. Diabetes Technol Ther. 2021;23:1–7.
Herzig D, Dehais J, Prost JC, et al. Pharmacokinetics of faster and standard insulin aspart during fully closed-loop insulin delivery in type 2 diabetes. Diabetes Technol Ther. 2020;22:691–6.
Bally L, Herzig D, Ruan Y, et al. Short-term fully closed-loop insulin delivery using faster insulin aspart compared with standard insulin aspart in type 2 diabetes. Diabetes Obes Metab. 2019;21:2718–22.
Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603.
Acknowledgements
We thank all investigators, trial staff and participants of this study.
Funding
Study design, data collection, analysis, and interpretation, as well as the journal’s Rapid Service Fee, were funded by Novo Nordisk A/S.
Medical Writing and Editorial Assistance
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by Matthew Robinson MChem DPhil and Helen Marshall BA, of Ashfield MedComms, and funded by Novo Nordisk A/S.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Steven J. Russell was the principal investigator of this clinical trial and the guarantor of this work and, as such, had full access to the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Magnus Ekelund was the medical specialist for the trial and had medical responsibility on a clinical-trial level. Tina Graungaard was the responsible statistician. Steven J. Russell, Courtney Balliro, Magnus Ekelund, Firas El-Khatib, Tina Graungaard, Evelyn Greaux, Mallory Hillard, Rabab Jafri, Naveen Rathor, Raj Selagamsetty, Jordan Sherwood, and Edward R. Damiano had access to the study data, take responsibility for the accuracy of the analysis, contributed to data interpretation, reviewed and contributed to the content of the manuscript, and had authority in the decision to submit the manuscript. All authors approved the manuscript for publication.
List of Investigators
The full list of site investigators is presented in Table S1 in the Supplementary Material.
Prior Presentation
Parts of this paper were presented in an abstract for the Advanced Technologies & Treatments for Diabetes (ATTD) meeting 2020, Madrid, Spain, 19–22 February 2020.
Disclosures
Steven J. Russell is an inventor on patents related to the iLet technology that are assigned to Massachusetts General Hospital and are licensed to Beta Bionics, and honoraria/travel/consultancy/grant from Novo Nordisk, Roche, Ascensia, Unomedical, Companion Medical, Beta Bionics, Senseonics, Flexion Therapeutics, Zealand Pharma, Adocia, and Tandem Diabetes. Courtney Balliro has travel/consulting from Novo Nordisk and Beta Bionics, Inc. Edward R. Damiano is affiliated with the Biomedical Engineering Department at Boston University (as a professor of Biomedical Engineering on an academic leave of absence). At the time of writing this manuscript, Firas El-Khatib and Raj Selagamsetty were affiliated with the Biomedical Engineering Department at Boston University; their current affiliation is with Beta Bionics, Inc. Concord, MA, USA. Edward R. Damiano and Firas El-Khatib are employees, co-founders, and equity holders in Beta Bionics, Inc. Edward R. Damiano serves on the Board of Directors of Beta Bionics, Inc. Raj Selagamsetty is an employee of, and holds options to purchase stock in, Beta Bionics, Inc. Firas El-Khatib, Edward R. Damiano, and Raj Selagamsetty are inventors on patents related to the iLet technology, which are assigned to Boston University and licensed to Beta Bionics, Inc. Magnus Ekelund, Tina Graungaard, and Naveen Rathor are employees of Novo Nordisk A/S. Magnus Ekelund and Tina Graungaard hold shares in Novo Nordisk A/S. Evelyn Greaux, Mallory Hillard and Rabab Z. Jafri have no conflicts of interest. Jordan Sherwood acknowledges funding from NIH training grant T32DK007028.
Compliance with Ethics Guidelines
This trial was conducted in accordance with the Declaration of Helsinki of 1964 and its later amendments, International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice, International Organization for Standardization 14155 and with US Food and Drug Administration 21 Code of Federal Regulations 312.120. The trial protocol, informed consent, and other relevant documents were reviewed and approved by local health authorities and an institutional review board (Partners Human Research Committee, ref. IRB00010760). Further details are provided in Table S1 in the Supplementary Material. All participants provided informed consent to participate in the study and publication of their clinical data for research purposes.
Data Availability
The datasets generated during and/or analyzed during this are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Russell, S.J., Balliro, C., Ekelund, M. et al. Improvements in Glycemic Control Achieved by Altering the tmax Setting in the iLet® Bionic Pancreas When Using Fast-Acting Insulin Aspart: A Randomized Trial. Diabetes Ther 12, 2019–2033 (2021). https://doi.org/10.1007/s13300-021-01087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01087-x