Abstract
Introduction
There is no consensus on the optimal therapeutic approach to adopt in patients with newly diagnosed type 2 diabetes mellitus (T2DM) to prevent cardiovascular disease (CVD). The study aimed to gather an expert consensus on the hypoglycemic treatment and CV risk management in patients with newly diagnosed T2DM through the Delphi methodology.
Methods
To address this issue, a list of 30 statements concerning the definition of “early T2DM patient”, early treatment, CV risk in T2DM, treat-to-benefit approach, and indications for treatment with glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose co-transporter 2 (SGLT2) inhibitors was developed. Using a two-round Delphi methodology, the survey was distributed to 80 Italian diabetes specialists who rated their level of agreement with each statement on a 5-point Likert scale. Consensus was predefined as more than 66% of the panel agreeing/disagreeing with any given statement.
Results
A total of 27/30 statements achieved consensus. A patient was defined as “early” according to pathophysiological or clinical interpretation, and/or the timing of the diagnosis. There was agreement on the importance to reach the lowest possible HbA1c level, since diagnosis, also using combination therapy with hypoglycemic drugs with a proven CV benefit. There was a consensus that a treat-to-benefit approach involves the addition of a glucose-lowering agent with proven CV benefits to metformin since diagnosis. The use of GLP-1RAs and SGLT2 inhibitors was considered a key strategy in this approach and the benefits were recognized also for patients with T2DM without established CVD. GLP-1RAs should be used at an earlier stage than SGLT2 inhibitors to prevent CVD, especially in patients with evidence of subclinical atherosclerotic disease.
Conclusion
This Delphi consensus recognized the importance to adopt a tailored hypoglycemic treatment of patients with T2DM according to their CVD risk and the key role of glucose-lowering agents with proven CV efficacy, GLP-1RAs and SGLT2 inhibitors, in the context of an early treat-to-benefit approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study |
Current guidelines and consensus recommend the use of hypoglycemic drugs with proven CV benefit for patients with type 2 diabetes (T2DM) and established CVD, heart failure, and/or chronic kidney disease. |
Clear recommendations for the treatment of patients with newly diagnosed T2DM to prevent cardiovascular disease are not yet available. |
The study aims to provide expert opinion on the hypoglycemic treatment and CV risk management in patients with newly diagnosed T2DM. |
What was learned from the study? |
Italian diabetologists consider the importance of adopting a personalized approach for patients with T2DM and CV risk factors. |
GLP-1RAs and SGLT2 inhibitors were recognized as key strategies in the treat-to-benefit approach. |
A paradigm shift should be implemented to focus clinicians’ attention not only on metabolic control but also on the long-term CV benefits, even at early stages. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14135225.
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive disease characterized by overt hyperglycemia, due to insulin resistance and various degree of beta cell dysfunction. However, the pathophysiology of T2DM is complex, involving several organs and apparatus, including the cardiovascular system [1]. Several of these changes may occur long before T2DM diagnosis, being already detectable in prediabetes states and healthy siblings of subjects with T2DM [2, 3].
Current local [4] and international diabetes guidelines recommend an HbA1c target of less than 6.5% for patients with T2DM with a short duration of disease, without comorbidities, and/or with long life expectancy [5, 6]. However, there is no consensus on the best therapeutic strategy to reach HbA1c goals in these patients. A stepwise treatment intensification has been the standard approach to achieve glycemic control using metformin monotherapy as the preferred first-line therapy for the treatment of patients with a recent diagnosis of T2DM, followed by treatment intensification to achieve HbA1c targets [7]. Several glucose-lowering agents can effectively reduce glucose levels, without the risk of hypoglycemia [8]. Furthermore, for some of them, additional beneficial effects on cardiovascular disease (CVD) risk factors and on major adverse cardiovascular events (MACE) have been demonstrated. In particular, in the cardiovascular outcomes trials (CVOTs), liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, dapagliflozin, and canagliflozin were demonstrated to reduce MACE, although with some differences [9,10,11,12,13,14,15,16,17,18,19].
Accordingly, the Italian, as well as international, guidelines recommend the use of glucagon-like peptide 1 receptor agonists (GLP-1RAs) and sodium-glucose co-transporter 2 (SGLT2) inhibitors in patients with a previous CVD event, heart failure (HF), and/or chronic kidney disease (CKD) [4, 5, 7, 8]. Notably, the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) study demonstrated the CVD beneficial effects of GLP-1RAs also in patients at relatively lower CV risk [12].
While for patients with high CV risk, established CVD, HF, or CKD the recommended second-line therapy consists in the addition of a glucose-lowering agent with proven CV benefit, such as an SGLT2 inhibitor or a GLP-1RA, the second-line treatment to recommend for subjects with T2DM but without overt CV/CKD/HF is still debated [7].
In this context, several clinical trials have demonstrated that the early combination therapy with an innovative glucose-lowering agent in addition to metformin can significantly increase the number of patients achieving HbA1c targets compared to the respective monotherapies, without increasing the incidence of hypoglycemia [20,21,22,23].
Moreover, increasing benefits on glucose control and weight management have been recently reported with the early introduction of glucose-lowering therapies with proven CV benefits, i.e., SGLT2 inhibitors and GLP-1RAs, simultaneously or soon after the failure with metformin [7, 21,22,23,24].
With this background in mind, the purpose of the study was to perform a Delphi survey among a panel of Italian diabetes specialists to gather an expert consensus on the treatment of patients with newly diagnosed T2DM, as well as generate expert ideas on the optimal early CV risk management in this population.
Methods
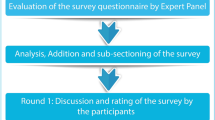
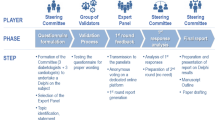
The Delphi method is a structured technique aimed at obtaining by repeated rounds of questionnaires a consensus opinion from a panel of experts in areas wherein evidence is scarce and opinion is important [25,26,27]. In the present manuscript, the consensus process consisted of a two-step web-based Delphi method, which took place between June and October 2020.
The survey was developed by a panel of eight physicians (four diabetologists, four cardiologists), identified as key opinion leaders (KOLs) in their respective fields in Italy. The KOLs met to fully analyze the published literature and discuss the unmet needs about the topic. Hence, they identified 30 statements with a major need of clarification and debate, focused on the definition of “early T2DM patient”, early treatment, CV risk, treat-to-benefit approach, and therapeutic strategies as an add-on to metformin. The panel also chose four Italian expert diabetologists to serve as external validators of the questionnaire, to test its understandability and clarity. After approval by the external validators, the questionnaire was distributed to 80 expert diabetologists via an online platform with anonymized results. The panelists were clinicians with solid experience in the field of diabetes (at least 10 years of clinical experience in diabetology), selected throughout the country mainly among regional presidents or vice presidents of the principal scientific societies in the field (Italian Diabetes Society, Association of Medical Diabetologists, Italian Society of Endocrinology, and Italian Society of Gerontology and Geriatrics) and members of the aforementioned Italian scientific societies. Furthermore, the size of the panel of Italian experts was determined by involving expert diabetologists from all Italian regions (from at least 1–12 diabetologists) to have a representative sample of the whole national territory and a homogeneous distribution between Northern, Central, and Southern Italy.
Panelists were invited to express their level of agreement or disagreement on each statement using a 5-point Likert scale, scored from 1 to 5 (1, strongly disagree; 2, disagree; 3, agree; 4, mostly agree; and 5, strongly agree). Results were expressed as a percentage of respondents who scored each item as 1 or 2 (disagreement) or as 3, 4, or 5 (agreement) (see electronic supplementary material). A positive consensus was reached in case of more than 66% agreement, a negative consensus in case of more than 66% disagreement, consensus was not reached when the sum for disagreement or agreement was below 66% [26, 27].
For the statements on which consensus had not been achieved, panelists were asked to re-rate in a second round their agreement/disagreement, after being provided with relevant literature on the topic selected by the KOLs in a dedicated meeting.
Descriptive analysis was performed to summarize the results.
Compliance with Ethics Guidelines
The study is based on a survey that does not involve the participation of human subjects nor patient data management and does not aim to modify the current clinical practice of participants. Consequently, this study did not require ethical approval. All experts involved in the Delphi survey were informed of the study’s objectives and the possibility of publishing the results in a peer-reviewed article. The participation was voluntary. They expressed their consent to participate in the survey after logging into the secure online survey platform via credentials, by actively clicking on the appropriate box.
Results
Degree of Consensus in the Delphi Process
In the first round of the Delphi survey, there were 60 respondents out of 80 invited panelists. Round 2 was completed by the 55 panelists who responded to round 1. Overall, the response rate was 69%: 64% of the respondents were female and the mean age was 53 years with a nationwide homogeneous distribution (Table 1). In round 1, consensus was reached for 25/30 statements (83%) (Fig. 1). In the second round, performed on the five statements for which consensus had not been reached, consensus was reached for 2/5 statements. Overall, 27 statements of the Delphi survey reached consensus (90%), while no consensus was reached for 3 statements (Fig. 1).
Table 2 summarizes the statements and presents the percentage of agreement/disagreement for each one based on the responses of the 55 panelists.
Major statements, grouped for macro-areas, are reported below.
“Early T2DM Patient”
The panelists strongly agreed that a patient is defined as “early” according to the short duration of disease (91%). Experts also agreed that the definition of the “early patient” is based on the absence of organ damage (73%); patients naïve to glucose-lowering therapy or treated with metformin alone were also considered “early” (73%).
Early Treatment
A consensus was reached regarding the importance to reach the lowest possible level of HbA1c, soon after the clinical diagnosis (95%). Furthermore, HbA1c targets should be reached using several glucose-lowering agents with at least one able to also reduce CV risk (75%). There was consensus that the reduction in CV risk is not strictly related to the reduction of HbA1c levels (82%), but the choice to use GLP-1RAs or SGLT2 inhibitors is not strictly related to the need to normalize HbA1c values (80%).
Definition of Cardiovascular Risk in T2DM
As for the distinction between primary and secondary CV prevention, there was consensus on the distinction based on a previous CV event (84%). Furthermore, panelists strongly disagreed that the definition of CV risk is based only on established CVD (i.e., a prior myocardial infarction) (98%). No consensus was reached on whether the distinction should be based on the level of CV risk or if the definition of CV risk is based on the presence of obliterating arteriopathy of the lower limbs.
Consensus on the CV risk of patients with T2DM was almost unanimous. Panelists agreed that patients may have different levels of CV risk (91%) and that the definition of a high-risk diabetic patient may be independent of the duration of the disease (100%).
A consensus was also reached on the statement that urinary albumin/creatinine ratio, a well-known marker of diabetic kidney disease, is a strong predictor of CVD (91%).
Treat-to-Benefit Approach
When the treat-to-benefit approach was considered, there was a strong consensus for the early addition of a second glucose-lowering agent with proven CV benefits (98%). Panelists unanimously agreed that the choice of GLP-1RAs and SGLT2 inhibitors represents a key strategy in this approach (100%).
Furthermore, treatment with metformin could not be necessarily considered the first choice in a patient with newly diagnosed T2DM and with concomitant CVD (76%). Besides, panelists agreed that sulfonylureas have a relatively small place in the therapeutic algorithm for the treatment of T2DM (76%), specifically in high-risk patients.
When criteria to prescribe a glucose-lowering agent in add-on to metformin were investigated, there was positive consensus that the choice should be made on the level of CV risk (85%), and on the presence of microangiopathy (76%). No consensus was obtained on the decision to prescribe a glucose-lowering agent in add-on to metformin based only on the presence of a previous CV event.
Indications for GLP-1RAs and SGLT2 Inhibitors
The expert panel agreed that the benefits of GLP-1RAs and SGLT2 inhibitors are not limited to patients with T2DM and CVD (95%) and that GLP-1RAs are more indicated in patients with high levels of HbA1c (76%). Furthermore, panelists agreed to use GLP-1RAs at an earlier stage than SGLT2 inhibitors to prevent atherosclerotic disease (76%) but not HF (84%). There was also a strong consensus on the use of GLP-1RAs as the first choice over SGLT2 inhibitors in patients with evidence of subclinical atherosclerotic disease (96%). Panelists disagreed that GLP-1RAs and SGLT2 inhibitors should be used only in patients with a subclinical atherosclerotic disease (96%). There was consensus that the prevalence (69%) and the risk (71%) of atherosclerotic CVD are higher than those of HF in patients with T2DM. Finally, consensus on the beneficial effects of GLP-1RAs and SGLT2 inhibitors on multiple CV risk factors was unanimous (100%).
Discussion
The purpose of the study was to perform a Delphi survey among a panel of Italian diabetes specialists to gather an expert consensus on the treatment of patients with newly diagnosed T2DM, as well as to generate clinical recommendations on the optimal management of CV risk immediately after the clinical diagnosis. After two rounds of Delphi survey, a consensus was reached on 27/30 statements.
Overall, panelists agreed that patients with newly diagnosed T2DM, without long-term complications, and with a preserved beta cell function may be considered “early” subjects. In this population the normalization of HbA1c should be sought aggressively, also by a combination therapy with agents with complementary mechanisms of action. To implement a treat-to-benefit approach, i.e., to reach an HbA1c at target and to decrease the CV risk, the early use of GLP-1RAs and SGLT2 inhibitors either as first- or second-line treatment in “early T2DM subjects” reached a broad consensus. The preference for one over the other of these classes of drugs is based on specific evidence-based characteristics, including the results of CVOTs and the recommendations of current consensus and guidelines in T2DM [6,7,8,9,10,11,12,13,14,15, 24].
Panelists also agreed on the distinction between primary and secondary CV prevention based on prior CV event and not on the level of CV risk. The Delphi consensus suggested that the glucose-lowering approach either for high CV risk or for very high-risk patients should be similar. The presence of microangiopathy, and in particular of diabetic kidney disease, was also unanimously considered an important risk factor for the definition of CVD risk.
These statements opened up several further considerations on the definition of the target patient to be considered “early”, the best pharmacological approach to treat this patient, and how to fill the gap in current recommendations.
According to Italian diabetes experts, a patient is defined as “early” according to different interpretations: pathophysiological and clinical aspects, and/or according to the timing of the diagnosis. These definitions identify a patient with newly diagnosed T2DM, drug naïve, or already treated with metformin alone, with no evidence of organ damage and, possibly, in an early stage of the disease, in which beta cell insulin secretion still sufficiently copes with insulin resistance.
However, T2DM may be diagnosed soon after the occurrence of the first CV event. Thus, the UKPDS, enrolling newly diagnosed patients with T2DM, highlighted that more than 50% of patients already had complications at diagnosis [28]. The presence of CVD at diagnosis was already documented in observational studies [29], in patients with diabetes or prediabetes [30].
The unanimous consensus on the need to reach the lowest possible level of HbA1c and normalize HbA1c is supported by several pieces of evidence. The results of the observational long-term follow-up of the UKPDS demonstrated that intensive glucose control starting at the time of diabetes diagnosis could be associated with a significantly decreased risk of myocardial infarction and death from any cause [31]. Also, the meta-analysis of CVOTs revealed that intensive glycemic control was associated with a 9% reduction in the risk of MACE [32], but not of all-cause or CV death.
The normalization of HbA1c at an early stage of the disease leads to a reduction in CV events [5, 31]. A population-based study on patients with newly diagnosed T2DM showed that a 1-year delay in treatment intensification in conjunction with poor glycemic control significantly increased the risks of HF, stroke, and composite CV events in patients with and without a history of CVD before diabetes diagnosis [33]. Furthermore, the achievement of an HbA1c less than 6.5% within 6 months after metformin initiation translated into a lower risk of CV events and death [34]. Thus, early glycemic control, particularly during the first year following diagnosis, may be crucial for the prevention of disease progression and of later complications [31, 35]. Furthermore, the legacy effect of early blood glucose control can extend up to almost 20 years after the clinical diagnosis of diabetes [36].
Panelists also agreed on the early use of a combination therapy to reach HbA1c targets and prevent CV risk. The concept of using multiple drugs immediately after the diagnosis of diabetes is relatively new in diabetology and diabetes guidelines have recently introduced, in the therapeutic algorithm, the possibility of initial combination therapy for newly diagnosed patients with HbA1c levels more than 1.5% above their target [7].
The VERIFY study demonstrated the potential of early combination therapy in providing greater and durable long-term benefits: time to loss of glycemic control was nearly doubled, and more than twice the number of patients experienced extended glycemic control, with a vildagliptin–metformin combination therapy versus metformin alone [37]. Similarly, a meta-analysis of 36 studies on the efficacy of initial combination therapy in drug-naïve patients with T2DM demonstrated that all initial combination therapies resulted in significant HbA1c reductions compared with metformin monotherapy [38].
Furthermore, early combination therapy using agents targeting different physiological abnormalities could provide longer periods with stable HbA1c levels, delaying the need for therapy intensification and reducing the risk of chronic complications [20]. The incoming GRADE (Glycemia Reduction Approaches in Diabetes: a Comparative Effectiveness) study will additionally clarify the clinical effectiveness of the addition of four classes of glucose-lowering agents to metformin [39].
Recent guidelines recommend routinely perform a CV risk assessment in T2DM [40] since diagnosis. ESC guidelines classified patients with T2DM in different CV risk classes, independent of baseline HbA1c, considering only patients aged less than 50 years with diabetes duration less than 10 years and no additional risk factors, at moderate risk. The presence of CVD, or target organ damage, or multiple major risk factors classifies the subject as very high risk [41]. This classification is in line with the consensus obtained on the independence of the definition of high-risk patients with T2DM from diabetes duration when CV events, organ damage, or multiple major risk factors are present.
Conversely, the statement that the definition of CV risk is based on the presence of peripheral artery disease (PAD) did not reach a consensus, because CV risk is multifactorial, and the presence of PAD is just one of the multiple criteria.
The perception of the importance of the evaluation of urinary albumin/creatinine ratio is also underlined by the ESC/EASD guidelines: there is a strong recommendation to routinely assess microalbuminuria to identify patients at risk of developing renal dysfunction or at high risk of CVD [24].
Notably, panelists strongly agreed on adopting a treat-to-benefit approach, a consensus that is in line with current guidelines that recommend glucose-lowering agents with proven CV benefit in people with T2DM and established CVD or at high/very high CV risk [7, 24]. The unanimous consensus on the use of GLP-1RAs and SGLT2 inhibitors in the treat-to-benefit approach reflects the awareness and knowledge of the multiple supporting evidence of these two agents in the reduction of CV events or kidney disease, associated with low risk of hypoglycemia.
Thus, the results of this Delphi consensus suggest early use of these classes of drugs, further extending the indications of current ESC/EASD guidelines that recommend GLP-1RAs or SGLT2 inhibitors in drug-naïve patients with established atherosclerotic CVD or those at high or very high risk (diabetes duration at least 10 years without target organ damage plus any other additional risk factors) [24].
However, despite these recommendations, only 15.3% of Italian patients with T2DM are currently treated with a glucose-lowering agent with an approved CV indication, irrespective of CV status [42]. This is a very low percentage when compared with the data from a diabetes register in Scotland, showing that more than 70% of naïve or metformin-treated patients with T2DM were at high or very high risk according to these guidelines [43].
The lack of consensus on the addition of a glucose-lowering-agent in add-on to metformin according to a previous CV event could be explained as diabetes specialists decide on the addition of another agent as an add-on to metformin mainly on the basis of glycemic control, considering several clinical aspects, including CV risk and kidney function.
Conversely, Italian real-world data from the ARNO Observatory showed that sulfonylureas are still used in 22% of patients with T2DM [44], a therapeutic approach that received a negative consensus in the Delphi survey in 76% of cases. The partially discordant agreement on this statement may stem from current guidelines that still allow use of sulfonylureas as third-line therapy [7].
In this regard, the lack of consensus on the addition of a glucose-lowering-agent in add-on to metformin according to a previous CV event was expected, and it is likely related to the complex clinical reasoning on the choice of second hypoglycemic agents after metformin that takes into account several clinical and non-clinical variables, including glucose control, diabetes duration, comorbidities, age, life expectancy, family support, etc.
The consensus reached on the choice of timing for introduction of GLP-1RA or SGLT2 inhibitors in the history of the disease reflects the acknowledgment of their demonstrated mechanisms of action and the results of clinical trials, including CVOTs. Thus, SGLT2 inhibitors, by acting through diuretic and natriuretic effects, decrease HF hospitalization, reduce CV mortality, and mitigate the progression of diabetic kidney disease [45], whereas GLP-1RAs contribute to risk reduction by both correcting multiple CV risk factors and by acting directly on the pathophysiology of atherosclerotic plaque [46]. The reduction of CV events obtained with SGLT2 inhibitors or GLP-1RAs is most likely independent of their glucose-lowering properties [47, 48].
The agreement on the use of SGLT2 inhibitors earlier than GLP-1RAs to prevent HF is supported by cumulative evidence from randomized controlled trials that demonstrated the efficacy of this class of drugs in reducing hospitalizations for HF by 23% and the risk of progression of renal disease by 45%. These effects were observed consistently across a range of estimated glomerular filtration rates (eGFRs) and irrespective of the presence of ischemic heart disease or HF at baseline [49, 50]. Although the mechanisms of the beneficial effects of SGLT2 inhibitors in cardiorenal complications are not fully understood [51], recent studies indicate that the renoprotective effects related to the natriuresis are involved in the improvement in hospitalization for HF [52, 53].
Recent data from the DAPA-HF [54] and EMPEROR-reduced [55] trials in patients with HF and a reduced ejection fraction with or without diabetes showed that patients treated with SGLT2 inhibitors had a lower risk of worsening HF or CV death than those on placebo, suggesting that SGLT2 inhibition is beneficial even in the absence of diabetes. On the other hand, GLP-1RAs showed no clear benefit on HF hospitalization reduction and there is uncertainty on GLP-1RA efficacy in patients with HF with reduced ejection fraction. Preliminary hypotheses suggest that these agents may be efficacious in patients with HF with preserved ejection fraction [56].
The results of the REWIND trial [12], which included a greater proportion of patients with T2DM without established CVD and with high CV risk, highlighted the efficacy of dulaglutide in the reduction of major CV events also in patients without a history of CVD. On the other hand, a clear benefit was not documented with dapagliflozin in the DECLARE-TIMI 58 study [14] for the same outcome. In this regard, the consensus obtained in the preference for GLP-1RAs in patients with the subclinical atherosclerotic disease may reflect these results from the CV outcomes trials as well as the documented mechanisms of action of the two classes of drugs from experimental and preclinical studies [57, 58].
The beneficial effects of GLP-1RAs and SGLT2 inhibitors on multiple CV risk factors have been well documented including advantages with respect to body weight, blood pressure, lipids, uric acid, and inflammatory markers, with some differences between the two classes in terms of efficacy on selected risk factors [58, 59]; accordingly, these beneficial effects were acknowledged by the Delphi panelists.
Notably, since this Delphi-driven consensus aimed to define the optimal early antidiabetic treatment for CVD prevention in patients with T2DM, only glucose-lowering agents with proven CVD benefits, i.e., GLP-1Ras and SGLT2i, were included; other classes of glucose-lowering agents with proven efficacy on glucose control, such as DPP4i, glitazones, and acarbose, were not taken into account.
There are some limitations to our study. Thus, even if consensus is reached results are dependent on the composition of the respondents. However, to minimize the potential for selection bias, panelists were identified according to their long-term experience in diabetes fields and to their nationwide distribution.
Moreover, the statements suggested by KOLs were further validated by four external expert validators, but the clarity of some of the statements might have been missed. The attrition rates over the two rounds were low, thereby ensuring that the range of expert opinion was adequately represented, and the level of consensus was specified a priori.
Conclusions
The results of the Delphi survey suggest that Italian diabetologists recognized the pivotal importance of adopting a tailored approach for patients with T2DM and CV risk factors; moreover, they emphasized the strategic role of the early treatment with GLP-1RAs and SGLT2 inhibitors in the treat-to-benefit approach.
A paradigm shift to enhance treatment adherence should therefore be implemented by focusing the attention not only on metabolic control but also on the long-term benefits of glucose-lowering agents with proven CV efficacy.
There are still some aspects where consensus was not achieved, reflecting open issues yet to be addressed.
References
Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95.
Vistisen D, Witte DR, Brunner EJ, et al. Risk of cardiovascular disease and death in individuals with prediabetes defined by different criteria: The Whitehall II study. Diabetes Care. 2018;41:899–906.
Pontiroli AE, Monti LD, Pizzini A, Piatti P. Familial clustering of arterial blood pressure, HDL cholesterol, and pro-insulin but not of insulin resistance and microalbuminuria in siblings of patients with type 2 diabetes. Diabetes Care. 2000;23:1359–64.
AMD-SID Standard Italiani per la cura del diabete mellito 2018 [Italian]. https://aemmedi.it/wp-content/uploads/2009/06/AMD-Standard-unico1.pdf. Accessed 21 January 2021.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2019. Diabetes Care. 2019;42[Suppl 1]:S61–S70.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2019 executive summary. Endocr Pract. 2019;25:69–100.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43(2):487–493. Erratum in: Diabetes Care. 2020;43:1670.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S111–S124.
Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22.
Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44.
Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29.
Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Nreu B, Dicembrini I, Tinti F, et al. Major cardiovascular events, heart failure, and atrial fibrillation in patients treated with glucagon-like peptide-1 receptor agonists: an updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30:1106–14.
Bonora BM, Avogaro A, Fadini GP. Effects of exenatide long-acting release on cardiovascular events and mortality in patients with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2019;56:1051–60.
Kristensen SL, Rørth R, Jhund PS, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–85.
Castellana M, Procino F, Sardone R, Trimboli P, Giannelli G. Generalizability of sodium-glucose co-transporter-2 inhibitors cardiovascular outcome trials to the type 2 diabetes population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19:87.
Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16:410–7.
Hadjadj S, Rosenstock J, Meinicke T, et al. Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care. 2016;39:1718–28.
Rosenstock J, Chuck L, Gonzalez-Ortiz M, et al. Initial combination therapy with canagliflozin plus metformin versus each component as monotherapy for drug-naive type 2 diabetes. Diabetes Care. 2016;39:353–62.
Mu Y, Pan C, Fan B, et al. Efficacy and safety of linagliptin/metformin single-pill combination as initial therapy in drug-naive Asian patients with type 2 diabetes. Diabetes Res Clin Pract. 2017;124:48–56.
Grant PJ, Cosentino F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: New features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J. 2019;40:3215–17.
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15.
Giannarou L, Zervas E. Using Delphi technique to build consensus in practice. Int J Bus Sci Appl Manage. 2014;9:65–82.
Walker A, Selfe J. The Delphi method: a useful tool for the allied health researcher. Br J Therapy and Rehabilitation. 1996;3:677–81.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. Erratum in: Lancet 1999;354:602.
Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–20.
Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–7.
Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Turnbull FM, Abraira C, Anderson RJ, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–98.
Paul SK, Klein K, Thorsted BL, et al. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100.
Svensson E, Baggesen LM, Johnsen SP, et al. Early glycemic control and magnitude of HbA1c reduction predict cardiovascular events and mortality: population-based cohort study of 24,752 metformin initiators. Diabetes Care. 2017;40:800–7.
Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care. 2019;42:416–26.
Takao T, Matsuyama Y, Suka M, et al. Analysis of the duration and extent of the legacy effect in patients with type 2 diabetes: a real-world longitudinal study. J Diabetes Complicat. 2019;33:516–22.
Matthews DR, Paldánius PM, Proot P, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394:1519–29.
Cai X, Gao X, Yang W, et al. Efficacy and safety of initial combination therapy in treatment-naïve type 2 diabetes patients: a systematic review and meta-analysis. Diabetes Ther. 2018;9:1995–2014.
Nathan DM, Buse JB, Kahn SE, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36:2254–61.
Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–414. Erratum in: J Am Coll Cardiol. 2019;74:1428–29. Erratum in: J Am Coll Cardiol. 2020;75:840.
Emerging Risk Factors Collaboration, Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Annali AMD 2020. Diabete di tipo 2 [Italian]. https://aemmedi.it/wp-content/uploads/2020/10/Annali-nuova-versione-2020_3ok.pdf. Accessed 4 December 2020.
Caparrotta TM, Blackbourn LAK, McGurnaghan SJ, et al. Prescribing paradigm shift? Applying the 2019 European Society of Cardiology-led guidelines on diabetes, prediabetes, and cardiovascular disease to assess eligibility for sodium-glucose cotransporter 2 inhibitors or glucagon-like peptide 1 receptor agonists as first-line monotherapy (or add-on to metformin monotherapy) in type 2 diabetes in Scotland. Diabetes Care. 2020;43:2034–41.
Cineca. Osservatorio ARNO Diabete. Il profilo assistenziale della popolazione con diabete. Rapporto 2019. https://www.siditalia.it/clinica/linee-guida-societari/send/80-linee-guida-documenti-societari/5025-rapporto-arno-diabete-2019. Accessed 4 December 2020.
Scholtes RA, van Baar MJB, Lytvyn Y, et al. Sodium glucose cotransporter (SGLT)-2 inhibitors: do we need them for glucose-lowering, for cardiorenal protection or both? Diabetes Obes Metab. 2019;21(Suppl 2):24–33.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metab. 2020. https://doi.org/10.1016/j.molmet.2020.101102.
Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41:356–63.
Sharma A, Verma S. Mechanisms by which glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes. 2020;44:93–102.
Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur Heart J. 2018;39:363–70.
Brown E, Wilding JP, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expanding role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2020:1–32.
Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose cotransporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1845–55.
Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–72.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24.
Khan MS, Fonarow GC, McGuire DK, et al. Glucagon-like peptide 1 receptor agonists and heart failure: the need for further evidence generation and practice guidelines optimization. Circulation. 2020;142:1205–18.
Luconi M, Cantini G, Ceriello A, Mannucci E. Perspectives on cardiovascular effects of incretin-based drugs: from bedside to bench, return trip. Int J Cardiol. 2017;241:302–10.
Giorgino F, Caruso I, Moellmann J, Lehrke M. Differential indication for SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with established atherosclerotic heart disease or at risk for congestive heart failure. Metabolism. 2020;104:154045.
Newman JD, Vani AK, Aleman JO, et al. The changing landscape of diabetes therapy for cardiovascular risk reduction: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1856–69.
Acknowledgements
The study group includes diabetology experts according to the recommended Delphi procedures. The contributors to the survey validation are listed below:
Antonio Carlo Bossi, Bergamo; Fabio Broglio, Torino; Ilaria Dicembrini, Firenze; Andrea Giaccari, Roma.
List of the Panel of Experts of the Delphi Survey: Eugenio Alessi, Reggio Calabria; Immacolata Ambrosino, Bari; Elisabetta Armenise, Bari; Roberto Baratta, Catania; Marco Giorgio Baroni, Roma; Francesco Brescia, Bari; Natalia Busciantella, Camerino (MC); Riccardo Candido, Trieste; Giuliana Cazzetta, Bari; Roberta Celleno, Perugia; Angelo Cignarelli, Bari; Andrea Da Porto, Udine; Roberto Da Ros, Udine; Chiara Di Loreto, Perugia; Olga Disoteo, Milano; Alessandro Dodesini, Bergamo; Rosanna Donvito, Bari; Maria Antonietta Fois, Cagliari; Gloria Formoso, Pescara; Elisa Forte, Gaeta (LT); Vera Frison, Padova; Lucia Frittitta, Catania; Marianna Galetta, San Benedetto del Tronto (AP); Domenico Greco, Marsala (TP); Giovanna Gregori, Massa Carrara; Ida Mangone, Vimercate (MB); Giuliana La Penna, Pescara; Grazia Giovanna La Verghetta, Pescara; Annunziata Lapolla, Padova; Luigi Laviola, Bari; Frida Leonetti, Roma; Giuseppe Lepore, Bergamo; Filomena Lo Conte, Bari; Giuseppina Manzoni, Monza; Reggiani Giulio Marchesini, Bologna; Stefano Masi, Salerno; Maria Masulli, Napoli; Cesare Miranda, Pordenone; Monica Modugno, Bari; Francesco Mollo, Rovigo; Mario Luca Morieri, Padova; Paola Orsini, Livorno; Mario Parillo, Caserta; Carlo Pedicino, Campobasso; Silvia Perra, Monza; Paola Ponzani, Genova; Maria Chantal Ponziani, Novara; Rosa Anna Rabini, Ancona; Loredana Rizzo, Grosseto; Anna Maria Romano, Lecce; Alessia Scatena, Arezzo; Antonio Silverii, Firenze; Elisabetta Torlone, Perugia; Maria Grazia Vita, Bari; Francesca Zerbini, Monza.
Funding
Publication charges were covered by Ethos s.r.l. through a Novo Nordisk S.p.A. unconditional grant. Novo Nordisk S.p.A. did not influence and were not involved in data collection, interpretation, and analysis. No funding or sponsorship was received for this study.
Editorial Assistance
Editorial assistance was provided by Alessandro Lico of Ethos s.r.l., through a Novo Nordisk S.p.A. unconditional grant, for technical support during the manuscript submission process.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Giuseppina Russo has received personal fees for consulting, lectures, speakers bureaus, expert testimony from Novo Nordisk, Eli Lilly, AstraZeneca, Mundipharma, Sanofi, Boehringer Ingelheim. Matteo Monami has received speaking fees from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Sanofi, and Novartis and research grants from Bristol Myers Squibb. Gianluca Perseghin received personal fees from Novo Nordisk, Sanofi, AstraZeneca, Lilly, MSD, Mundipharma, Novartis, Boheringer Ingelheim, Pfizer, Intercept, PikDare. Angelo Avogaro received grant from Astazeneca and MundiPharma, and personal fee from Novo Nordisk, Astrazeneca, Boehringher, Neopharmed, Mundipharma, Servier, Allergan and Takeda. Pasquale Perrone Filardi reported consultancy or speaker fees from AstraZeneca, Bayer, Boehringer, Daiichi, BMS, Pfizer, Menarini, Sanofi, Servier, Novartis, Doc, Novo Nordisk, Eli Lilly, Sandoz, Amgen, MSD, Merck Serono. Michele Senni reports personal fees from Novartis, Abbott Medical, MSD, Bayer, Vifor, Boehringer, AstraZeneca, outside the submitted work. Claudio Borghi has nothing to disclose. Aldo Pietro Maggioni has received personal fees from Bayer, Fresenius, Novartis.
Compliance with Ethics Guidelines
The study is based on a survey that does not involve the participation of human subjects nor patient data management and does not aim to modify the current clinical practice of participants. Consequently, this study did not require ethical approval. All experts involved in the Delphi survey were informed of the study’s objectives and the possibility of publishing the results in a peer-reviewed article. The participation was voluntary. They expressed their consent to participate in the survey after logging into the secure online survey platform via credentials, by actively clicking on the appropriate box.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Russo, G., Monami, M., Perseghin, G. et al. The “Early Treatment” Approach Reducing Cardiovascular Risk in Patients with Type 2 Diabetes: A Consensus From an Expert Panel Using the Delphi Technique. Diabetes Ther 12, 1445–1461 (2021). https://doi.org/10.1007/s13300-021-01045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-021-01045-7