Abstract
A dietary protein intake (DPI) of between 0.6 and 0.8 g protein per kilogram body weight per day (g/kg/day) is frequently recommended for adults with moderate-to-advanced chronic kidney disease (CKD). However, evidence on whether patients with diabetic kidney disease (DKD) actually benefit from a DPI of ≤ 0.8 g/kg/day and from a low-protein diet (LPD) at CKD stages 1–3 has not been consistent. We systematically searched MEDLINE, EMBASE, Cochrane Library, Web of Knowledge, as well as the bibliographies of articles identified in the search, for eligible randomized controlled trials that had investigated the effects of LPD (prescribed DPI < 0.8 g/kg/day) versus control diet on the progression of DKD. Nine trials that included 506 participants and follow-up periods varying from 4.5 to 60 months were included in the subsequent systematic review and meta-analysis. The data showed that patients with DKD who consumed < 0.8 g protein/kg/day had a significantly reduced decline in glomerular filtration rate (GFR) (mean difference [MD] 22.31 mL/min/1.73 m2, 95% confidence interval [CI] 17.19, 27.42; P < 0.01) and a significant decrease in proteinuria (standard mean difference [SMD] − 2.26 units, 95% CI − 2.99, − 1.52; P < 0.001) versus those on the control diet. The benefits of LPD to patients with DKD at CKD stages 1–3 were a markedly decreased proteinuria (SMD − 0.96 units, 95% CI − 1.81, − 0.11; P = 0.03) and slight but significant decreases in glycated hemoglobin (− 0.42%) and cholesterol levels (− 0.22 mmol/L). Our meta-analysis indicated that a DPI of < 0.8 g/kg/day was strongly associated with a slow decline in GFR and decreased proteinuria in the patients with DKD. Patients with CKD stages CKD 1–3 benefited from LPD in terms of a marked decrease of proteinuria and slight but significant improvements in lipid and glucose control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Diabetic kidney disease (DKD) is a major complication of diabetes mellitus and the leading cause of end-stage renal disease in developed countries. Dietary management is one of the most important measures to delay the progression of DKD. |
Research has shown that dietary protein restriction slows down the progression of chronic kidney disease, but its role in DKD remains controversial. |
The aim of this study was to assess the evidence-based efficacy of a low-protein diet in the management of DKD? |
What was learned from the study? |
Our assessment of the efficacy of a low-protein diet in patients at different stages of DKD was based on evaluations of the glomerular filtration rate (GFR) and proteinuria. |
We found a strong association between a low-protein diet and both a slow decline in GFR and decrease in proteinuria in the patients with DKD. |
Patients at CKD stages 1–3 benefited from LPD with a marked decrease in proteinuria and a slight but significant improvement in lipid and glucose control. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13095599.
Introduction
Diabetic kidney disease (DKD) is a major complication of diabetes mellitus (DM) and the leading cause of end-stage renal disease (ESRD) in developed countries [1]. In China, the past decade has seen a drastic increase in the prevalence of DM-related chronic kidney disease (CKD) compared to glomerulonephritis-related CKD [2]. The current recommended standards of medical care in DKD include diet therapy, optimal glucose, lipid and blood pressure control, inhibition of the renin–angiotensin–aldosterone system and sodium glucose co-transporter 2 inhibitor treatment, all of which have the aim to suppress the progression of DKD and decrease the risk for cardiovascular disease [3,4,5]. However, despite these treatments, some patients with early-stage DKD rapidly progress to ESRD. Consequently, effective therapeutic approaches are urgently needed.
There is an increasing body of evidence demonstrating that diet therapy, especially a low-protein diet (LPD), is a fundamental and safe treatment for suppression of DKD progression [5,6,7,8,9] and that a very low-protein diet (VLPD) may be even more beneficial in terms of renoprotection than a conventional LPD in patients with non-DKD [10]. Of the various levels of low-protein intake, the range of 0.6–0.8 g protein per kilogram body weight per day (g/kg/day) is the most frequently recommended target for adults with moderate-to-advanced CKD. However, studies on whether patients with DKD actually benefit from a dietary protein intake (DPI) of < 0.8 g/kg/day and from a LPD at early stages of CKD (CKD stages 1–3) have not produced consistent results.
Here, report our updated systemic review and meta-analysis in which we have included recent randomized controlled studies (RCTs). Our aim was to assess the effect of the standardly prescribed DPI ≤ 0.8 g/kg/day versus an unrestricted protein diet on renal function and metabolism in patients with DKD at different CKD stages.
Methods
Our study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11].
Data Sources and Literature Search
The MEDLINE, EMBASE, Cochrane Library and Web of Knowledge databases were searched for eligible RCTs that had investigated the effects of LPD (prescribed DPI < 0.8 g/kg/day) versus control diet on kidney function, proteinuria, glycemic control or nutritional status in patients with DKD before March 2020. The search terms used were: “Diet, protein-restricted” OR protein restrict* OR “low protein” AND “diabetic nephropathies” OR “diabetic nephropathy” OR “diabetic glomerulo” OR “([diabetic OR diabetes] AND [kidney disease OR renal disease])”. The detailed search strategy used for MEDLINE is shown in Electronic Supplementary Material (ESM) Table S1. The search was limited to studies performed on humans, and no language restriction was imposed. The bibliographies of studies identified in the search as eligible for inclusion in our meta-analysis were also searched manually for relevant studies. Two authors (QL Li and F Wen) independently screened the search records.
This research is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Study Selection
Agreement on the final inclusion of studies was reached by consensus. The inclusion criteria were: (1) DKD patients with type 1 or type 2 DM with a mean intervention period > 4 months; (2) intervention with a protein-restricted diet of ≤ 0.8 g/kg/day; (3) availability of outcome data for changes in glomerular filtration rate (GFR)/estimated GFR (eGFR) or creatinine clearance (CCr) and proteinuria. Intensive protein restriction was defined as a mean actual DPI ≤ 0.8 g/kg/day. Early DKD was defined as CKD stages 1–3. Studies were excluded if details of diet were not given or subjects had obvious hyperfiltration or intervention immediately before dialysis. Crossover studies were not included.
Data Extraction and Quality Assessment
Various data elements were extracted from the publications included in the systemic review and meta-analysis: (1) age, sex, country and type and duration of diabetes; (2) dietary nutritional composition and protein prescribed (low-protein intake or control), protein intake assessment methods and intervention period; and (3) primary (changes in GFR/eGFR or CCr, changes in proteinuria) and secondary outcomes (glycated hemoglobin [HbA1c], serum albumin, serum lipids, ESRD, life quality). GFR and CCr were used interchangeably because CCr was commonly used in the included studies as an estimate of GFR. Early intervention indicated that the GFR/eGFR or CCr of the enrolled population was > 30 mL/min/1.73 m2. Two authors (QL Li and F Wen) independently extracted key study characteristics. Any disagreement was resolved by discussion and consultation with other investigators in the research group.
Two authors (QL Li and F Wen) independently assessed the quality of and risk of bias in each included study using the Cochrane Collaboration’s tool to assess the seven types of bias (sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias). The overall quality of evidence was rated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach using GRADEpro software V.3.6.1 (for Windows; Jan Brozek, Andrew Oxman, Holger Schünemann, updated 2011).
Data Synthesis and Statistical Analysis
The standard deviation was calculated using the 95% confidence interval (CI) or interquartile range. The methods for calculating the change-from-baseline SD are referenced in the Cochrane Handbook version 5.1.0 part 16.1.3 (https://handbook-5-1.cochrane.org/). The correlation coefficient was obtained from other indicators of the same study or from a study giving detailed information. The meta-analysis was performed using Review Manager 5.3 (https://review-manager.software.informer.com/5.3/; The Cochrane Collaboration, Oxford, UK). First, a subgroup analysis was performed for the primary outcomes, such as actual protein intake (< 0.8, 0.8–1.0 or > 1.0 g/kg/day) and CKD stage at enrollment (CKD 1–3, CKD 1–5). Other subgroup analyses included types of diabetes, countries, intervention period, age and GFR measures. The difference in change in HbA1c between two groups was also analyzed. Meta-analyses of serum albumin and serum lipid series were compared before and after the LPD intervention. Most effects were pooled using a random-effects model, except for the effects of HbA1c that were pooled using a fixed-effects model. Heterogeneity was assessed using the I2 statistic. STATA software (version 12.0; Stata Corp., College Station, TX, USA) was used to perform the sensitivity analysis and calculate publication bias. Statistical significance was set at P < 0.05 for all analyses.
Results
Selected Studies and Characteristics
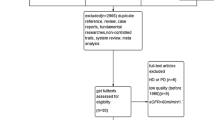
A flow diagram of the literature search process is shown in Fig. 1. Of the 763 articles retrieved from our search of the databases and bibliographies of the identified articles, 17 full-text articles were included for detailed assessments. Of these 17 articles, one study was subsequently excluded due to absence of comparison [12]; one study was excluded because the duration of the intervention was too short [13]; two studies were crossover designs and excluded [14, 15]; two studies used repeated results and were excluded [16, 17]; one study was excluded because it enrolled patients with obvious hyperfiltration (75% decrease in the GFR resulted from changes in the effective renal plasma flow [18]); and one study was excluded because the enrolled patients had worse renal function than CKD stages 1–3 based on the results that more than one-half of the patients (20/32) had entered dialysis or died during an average follow-up of 1.8 years [19]. The nine remaining studies were included in the quantitative analysis.
A detailed description of the characteristics of the included studies is given in Table 1. In total, 256 and 250 subjects were evaluated in the LPD group and the control group, respectively, with a mean intervention period ranging from 4.5 to 60 months. Two of the included studies focused on patients with type 2 DM; five focused on those with type 1 DM; and the remaining two studies included both types of DM patients. All included RCTs provided data on changes in GFR or CCr and in proteinuria in both the intervention and control groups. Two studies enrolled patients at CKD stage 3 [20, 21]; three studies did not focus on GFR inclusion criteria, rather they mostly focused on patients with CKD stages CKD 1–3 at baseline [22,23,24]; the other four studies made a relatively loose requirement of CKD between stages 1 and 4 [25,26,27,28]. Only one study included patients with normal albuminuria [24]. Six studies provided data on the changes in HbA1c. Three studies provided data on serum albumin before and after LPD intervention. Three RCTs noted serum lipid series. Only one study reported ESRD as an outcome [28]; another study analyzed life quality as an outcome [25].
Diet Compliance Assessment
All studies assessed dietary protein intake using 24-h urine urea nitrogen excretion as a measure and/or a food record or recall technique, following which the outcomes were analyzed statistically (Table 2). The prescribed amount of protein intake in the intervention group ranged from 0.6 to 0.8 g/kg/day. The average actual protein intake was 0.9 g/kg/day (range 0.71–1.1 g/kg/day) in the LPD group and 1.29 g/kg/day in the control group (P < 0.05). In only two studies did the patients reach a mean daily protein intake < 0.8 g/kg/day [20, 23], indicating poor patient compliance to the intervention. In contrast, in more than one-half of the studies (5/9) patients in the control groups had a lower mean protein intake (1.03–1.14 g/kg/day) than the expected levels (1.1–1.2 g/kg/day). Specifically, of the five studies which specifically provided data on the energy intake and/or nutrient contents of the diets [21,22,23, 25, 27], only one mentioned that there was a significant difference in energy intake during the study period [21]. No study restricted protein with a ketoanalgue supplement.
Effects of Intensive Protein Restriction on Renal Function
The decline in change in the weight mean difference (WMD) for GFR was statistically significantly different [WMD 3.86 mL/min/1.73 m2, 95% CI 0.55–7.17 mL/min/1.73 m2; P = 0.02; I2 = 89%] between the LPD and control groups (Fig. 2a). The heterogeneity was significant, but a slight asymmetry was found in the funnel plot (ESM Fig. S1). GFR was showed to be markedly improved in the subgroup of patients whose actual protein intake was < 0.8 g/kg/day (MD 22.31 mL/min/1.73 m2, 95% CI 17.19–27.42 mL/min/1.73 m2; P < 0.001; I2 = 0%) (Fig. 2a).
Effect of intensive protein restriction on the decrease in GFR (a) and proteinuria (b). Five different measures of proteinuria were used in the studies. Weights are from the random-effects analysis. P value < 0.05 indicates a significant difference. CI Confidence interval, IV weighted mean difference, GFR Glomerular filtration rate, LPD low-protein diet, SD standard deviation, Std. standard
The standard mean difference (SMD) was used to compare the effect of LPD on proteinuria because three different measures were used in the nine RCTs (Table 1). The change in the SMD for proteinuria was statistically significant and showed a decrease in 0.88 units in the LPD group (95% CI − 1.53 to − 0.24 units; P = 0.007; I2 = 90%) (Fig. 2b) compared to a marked improvement in those with an actual protein intake of < 0.8 g/kg/day (SMD − 2.26 units, 95% CI − 2.99 to − 1.52 units; P < 0.00001; I2 = 0%] (Fig. 2b). The funnel plot showed no major asymmetry (ESM Fig. S2).
Effects of LPD on Renal Function in Patients with DKD at Early Stages
The subgroup analysis of patients with CKD stages 1–3 found no statistical differences in GFR decline between the LPD and control groups (WMD 7.33 mL/min/1.73 m2, 95% CI − 1.61 to 16.27 mL/min/1.73 m2; P = 0.11; I2 = 94%) (Fig. 3a). However, the patients with DKD at CKD stages CKD 1–3 benefited from protein restriction by achieving a markedly decreased proteinuria (SMD − 0.96 units, 95% CI − 1.81 to − 0.11; P = 0.03; I2 = 90%) (Fig. 3b). The funnel plot showed no major asymmetry.
Effect of early protein restriction (during CKD stages 1–3) on GFR and proteinuria. Changes are expressed as the weighted mean difference for GFR (a) and standardized mean difference for proteinuria (b). Weights are from the random-effects analysis. P value < 0.05 indicates a significant difference. CKD Chronic kidney disease,
Subgroup Analysis
Actual protein intake (I2 = 98.6%), location (I2 = 63.1%) and intervention period (I2 = 60.5%) may be responsible for the heterogeneity observed during the subgroup analysis of the effects of LPD on GFR (ESM Table S2). Patients with DKD exhibited a markedly improved GFR in those subgroups of patients with actual protein intake < 0.8 g/kg/day, intervention period < 1 year or age < 60 years, or with populations from North America. However, no significant differences were observed between the subgroups when actual protein intake was > 0.8 g/kg/day (P > 0.05; ESM Table S3).
Subgroup analysis of the effect of LPD on proteinuria was also performed (ESM Table S4). The heterogeneity could be partly explained by actual protein intake (I2 = 92.2%), type of DM (I2 = 86.3%), intervention period (I2 = 86.4%) and age (I2 = 85.7%). Patients showed improvement in proteinuria in the subgroups with actual protein intake < 0.8 g/kg/day, type 1 DM, intervention period < 1 year, mean age < 60 years and GFR measured using radionuclide assay, and in studies from North America. Even in the group with actual protein intake > 0.8 g/kg/day, patients with an intervention period < 1 year and mean age < 60 years exhibited a reduced proteinuria compared with the control group (ESM Table S3).
Effects of LPD on Metabolism
The HBA1c level was used as the measure of glycemic level. Patients with DKD on a protein-restricted diet exhibited a slight but significanty decrease in HbA1c compared with the control group (WMD − 0.42%, 95% CI − 0.68 to − 0.17, P = 0.001) (Fig. 4a). Moderate heterogeneity was found across the studies (I2 = 55%). Three studies [21, 23, 26] mentioned a change in blood glucose level, and all of these reported no differences during the follow-up period. Only two studies [23, 27] described changes in insulin dose, with one reporting a slight decrease.
Patients with DKD who were on a protein-restricted diet showed a slight but significant decrease in cholesterol level (WMD − 0.22 mmol/L, 95% CI − 0.40 to − 0.03 mmol/L; P = 0.02) (Fig. 4b) compared with the control group. No heterogeneity was found across the studies for cholesterol (I2 = 0%). No signs of deterioration in nutritional status were noted in the LPD groups of the nine trials during the median follow-up time of 24 months (WMD − 5.16, 95% CI − 10.97 to 0.64 g/L; P = 0.08; I2 = 92%) (ESM Fig. S3). Nutritional status was mainly assessed by weight, body mass index, mid-arm circumference and pre-albumin status. No signs of malnutrition were noted in patients in the LPD groups in the nine trials.
Sensitivity Analysis
A sensitivity analysis was conducted in which each study was excluded separately from the analysis to specifically identify the effect of that study on the outcomes (ESM Fig. S4). Following exclusion of the study by Zeller et al. [20], the outcome of GFR decline was negative and the heterogeneity almost disappeared (WMD 0.20, 95% CI − 0.40 to 0.81 mmol/L; P = 0.51; I2 = 2%), while the outcome of proteinuria decline was still positive and the heterogeneity slightly decreased (SMD − 0.71, 95% CI − 1.34 to − 0.08 mmol/L; P = 0.03; I2 = 89%). However, the research design and follow-up of this trial met the requirements quite well. No evidence supported the notion of excluding this RCT from the final analysis. Excluding the only study that enrolled some patients with normal albuminuria did not change the earlier findings (P = 0.02; I2 = 90%).
Risk of Bias and Overall Quality of Evidence
Risk of bias of all included studies is shown in ESM Fig. S5 and S6. Randomization sequence generation was mentioned in two studies. One study allocated patients according to age, sex, GFR, and other categories. Two studies reported concealment of allocation. One study reported the blinding of outcome assessors, and in another study, the allocation was not known to the general practitioner or the laboratory personnel. One study reported an intention-to-treat analysis. All studies adequately reported withdrawals. The overall quality of evidence was low.
Publication Bias
Little asymmetry was evident in the funnel plot (ESM Figs. S1, S2). The weighted regression test indicated no statistical evidence of publication bias for GFR (bias 1.51; P = 0.174).
Discussion
Our analyses revealed that an actual DPI < 0.8 g/kg/day provided notable renoprotection in terms of DKD course by improving both GFR and proteinuria. In addition, patients actually maintaining LPD achieved a slight but significantly decrease in HbA1c and cholesterol levels without worsening nutritional status. Our data clearly show that the patients with DKD with CKD stages 1–3 who strictly restricted their protein intake benefited in achieving markedly decreased proteinuria.
The findings of our study are different from those of an earlier meta-analysis performed by Robertson et al. [29]. These latter authors reported that a LPD delays the progression of DKD to some extent, but not significantly, while we found that a LPD provided a marked renoprotection on the course of diabetic nephropathy by improving GFR and proteinuria. One possible explanation these different conclusions may be associated with the variations in the inclusion and exclusion criteria of the respective studies, in particular in the inclusion of certain studies. For example, there is some controversy surrounding inclusion of the two studies by Pijls et al. [17, 24] in the meta-analysis of Robertson et al. [29]. The two studies by Pijls et al. [17, 24] both involved patients with similar baseline demographic and clinical characteristics, but the more recent study [24] included a longer intervention period and a larger number of patients. In our opinion this made the more recent study an extension of the previous results and more complete; Due to a similar design, identical baseline characteristics and availability of data, we decided to include only the more recent study in our analysis.
Consistent with most other analyses of RCTs and several meta-analyses [7, 9, 30, 31], our data demonstrated that patients with DKD benefited from a diet characterized by intensive protein restriction. A sustained LPD dilates the efferent arterioles and decreases intraglomerular pressure, leading to an improvement in GFR. In addition, a lower intake of dietary protein results in inhibition of the glomerular hyperfiltration-stimulated mesangial-cell signaling that leads to increased transforming growth factor β release and subsequent progressive fibrosis and, ultimately, renal inflammation [32]. Importantly, LPD can be synergistic with the direct effect of a low-sodium diet as well as the effect of angiotensin pathway inhibition [8]. However, this effectiveness was observed only when the patients’ compliance to the diet was fair. It should be noted that although the average mean protein intake in the LPD group of the present study was significantly lower than that of the control group, the intervention groups still consumed approximately 20% excess protein than prescribed in the diet; only two studies reported reaching a mean daily intake < 0.8 g/kg/day [20, 23]. Thus, regular surveillance of DPI is important for assessment of the compliance of patients who on a LPD.
The recommended dietary allowance of protein for healthy persons is 0.8 g/kg/day; in comparison, the estimated average requirement for adults with CKD is 0.66 g/kg/day. Thus, a diet consisting of 0.6–0.8 g protein/kg/day fulfills dietary needs. Indeed, a VLPD (< 0.6 g/kg/day) supplemented with essential amino acids or their ketoacids is also prescribed for certain patients [10]. However, the protein intake in a normal diet is usually more than the recommended dietary allowance, especially patients with DKD, because a high-protein diet is a popular weight-reduction strategy in persons with diabetes [33]. For people with nondialysis-dependent DKD, a daily dietary protein allowance of < 0.8 g/kg/day is not recommended because it does not alter glycemic measures, cardiovascular risk measures or the course of GFR decline by guideline [4] according to Modification of Diet in Renal Disease study [34]. However, several relevant limitations of the MDRD study, such as the relatively small sample, exclusion of patients with DKD, discounting of racial disparities and failure to separate the temporary short-term versus more persistent long-term effects on GFR, should be noted [8]. By including more recent studies with longer intervention period, larger number of patients and a second analysis of MDRD study [21, 22], our aim was to extend data provided by previous studies.
Our analysis also provided evidence that patients with DKD possibly benefited from LPD as early as CKD stages 1–3. Current standards of medical management in DKD, including diet intervention, glucose and lipid control, inhibition of the renin–angiotensin–aldosterone system and use of sodium glucose co-transporter 2 inhibitors, are recommended strategies to suppress the progression of DKD and decrease the risk of cardiovascular disease [3,4,5]. Diet intervention, including a low-protein diet, plays a fundamental role in all aspects of the treatment of patients with CKD, including DKD. Kalantar-Zadeh and Fouque [8] reported that early diet therapy can decelerate the progression of early stages of CKD and may help delay the need for kidney replacement therapy in the advanced stage. In the KDOQI Clinical Practice Guideline [35, 36], a low-protein diet (0.6–0.8 g/kg/day) is recommended to patients with DKD. Similarly, in animal models and human kidney diseases, a LPD was reported to ameliorate glomerular hyperfiltration and proteinuria even in an early stage of kidney disease and in patients previously consuming a high-protein diet [8, 37]. Importantly, the combination of LPD–keto acid administration could slow or halt the progression of renal insufficiency, especially when the treatment is initiated early [10, 38,39,40]. However, most studies to date have focused on patients at CKD stages 3–4, and only a few studies followed up with an early stages of DKD for a short term of 3–4 weeks [41, 42]. Although a protective effect of LPD on residual renal function has been reported [19, 28], the assessment of a single effect of dietary restriction in patients with early DKD is complicated due to the high drop-out rate in previous studies [19, 43].
Regarding the safety of and adherence to a LPD, this study showed that patients with DKD benefited from LPD by achieving an additional improvement in glucose and cholesterol control without any worsening of nutritional status. Although related data, such as caloric intake and use of statins, were not provided in the studies included in our meta-analysis, the observed better glucose and lipid control was possibly related to the additional benefits of LPD because protein restriction concomitantly reduced caloric intake and improved insulin resistance in patients with CKD [44]. Meanwhile, the potential risk of protein-energy wasting of and adherence to a LPD can be improved by providing adequate energy (30–35 kcal/kg/day) [45] and ongoing nutritional education and surveillance [46].
The main limitations of the present study were the moderate number and size of the RCTs available for analysis and the heterogeneity of the participants in these trials. The measures of GFR and proteinuria or albuminuria varied because of a large range in the years the studies were published. The value of GFR change was not provided in each study. These differences may explain some of the heterogeneity. Second, most of the significant results reported here were related to the short-term effects of dietary intervention and were notable almost exclusively in younger patients, possibly accounting for the hemodynamic factors. At the early stage of DKD, patients suffered from hyperfiltration; however, LPD may cause changes in renal function measurements. Also, most of the trials included in this study included participants with additional CKD risk factors, including hypertension and hyperlipidemia, which also limits the generalizability of the findings. Moreover, great overlaps in CKD stage might disguise the real benefit for kidneys. Other factors, such as hypertension, hyperlipidemia, inflammation, malnutrition and proteinuria, have become major causes for the disease with the progression of DKD. Also, the dietary energy intake and its effect on LPD were not analyzed because the data were not available in the included studies. Although our analysis suggests that DPI < 0.8 g/kg/day is likely to achieve additional benefits for cardiovascular disease and blood glucose control, sufficient data are lacking to confirm a specific DPI threshold. A well-designed, large-scale and multicenter study is needed in the future.
Conclusion
This systemic review and meta-analysis provided evidence that DPI < 0.8 g/kg/day achieved a marked renoprotection on the course of diabetic nephropathy by improving GFR and proteinuria. Patients with DKD at CKD stages 1–3 benefited from strict protein restriction by achieving a markedly decreased proteinuria as well as an additional reduction of lipid and blood glucose levels without any worsening of nutritional status.
References
Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4):510–33.
Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–6.
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306.
American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S135–51.
Molitch ME, Adler AI, Flyvbjerg A, Nelson RG, So WY, Wanner C, et al. Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney Int. 2015;87(1):20–30.
Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982;307(11):652–9.
Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases. Ann Intern Med. 1996;124(7):627–32.
Kalantar-Zadeh K, Fouque D. Nutritional management of chronic kidney disease. N Engl J Med. 2017;377(18):1765–76.
Nezu U, Kamiyama H, Kondo Y, Sakuma M, Morimoto T, Ueda S. Effect of low-protein diet on kidney function in diabetic nephropathy: meta-analysis of randomised controlled trials. BMJ Open. 2013;3(5):e002934.
Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol. 2016;27(7):2164–76.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Evanoff GV, Thompson CS, Brown J, Weinman J, et al. The effect of dietary protein restriction on the progression of diabetic nephropathy. A 12-month follow-up. Arch Intern Med. 1987;147(3):492–5.
Velazquez Lopez LSAM, Goycochea Robles MV, Torres Tamayo M, Castaneda Limones R. Effect of protein restriction diet on renal function and metabolic control in patients with type 2 diabetes a randomized clinical trial. Nutr Hosp. 2008;23(2):141–7.
Barsotti G, Navalesi R, Giampietro O, et al. Effects of a vegetarian, supplemented diet on renal function, proteinuria, and glucose metabolism in patients with ‘overt’ diabetic nephropathy and renal insufficiency. Contrib Nephrol. 1988;65:87–94.
Walker JD, Dodds RA, Murrells TJ, et al. Restriction of dietary protein and progression of renal failure in diabetic nephropathy. Lancet. 1989;2(8677):1411–5.
Meloni C, Morosetti M, Suraci C, et al. Severe dietary protein restriction in overt diabetic nephropathy: benefits or risks? J Ren Nutr. 2002;12(2):96–101.
Pijls LT, de Vries H, Donker AJ, van Eijk JT. The effect of protein restriction on albuminuria in patients with type 2 diabetes mellitus: a randomized trial. Nephrol Dial Transplant. 1999;14(6):1445–53.
Dullaart RP, Beusekamp BJ, Meijer S, van Doormaal JJ, Sluiter WJ. Long-term effects of protein-restricted diet on albuminuria and renal function in IDDM patients without clinical nephropathy and hypertension. Diabetes Care. 1993;16(2):483–92.
Barsotti G, Cupisti A, Barsotti M, et al. Dietary treatment of diabetic nephropathy with chronic renal failure. Nephrol Dial Transplant. 1998;13(Suppl 8):49–52.
Zeller K, Whittaker E, Sullivan L, Raskin P, Jacobson HR. Effect of restricting dietary protein on the progression of renal failure in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1991;324(2):78–84.
Meloni C, Tatangelo P, Cipriani S, et al. Adequate protein dietary restriction in diabetic and nondiabetic patients with chronic renal failure. J Ren Nutr. 2004;14(4):208–13.
Dussol B, Iovanna C, Raccah D, et al. A randomized trial of low-protein diet in type 1 and in type 2 diabetes mellitus patients with incipient and overt nephropathy. J Ren Nutr. 2005;15(4):398–406.
Ciavarella A, Di Mizio G, Stefoni S, Borgnino LC, Vannini P. Reduced albuminuria after dietary protein restriction in insulin-dependent diabetic patients with clinical nephropathy. Diabetes Care. 1987;10(4):407–13.
Pijls LT, de Vries H, van Eijk JT, Donker AJ. Protein restriction, glomerular filtration rate and albuminuria in patients with type 2 diabetes mellitus: a randomized trial. Eur J Clin Nutr. 2002;56(12):1200–7.
Koya D, Haneda M, Inomata S, et al. Long-term effect of modification of dietary protein intake on the progression of diabetic nephropathy: a randomised controlled trial. Diabetologia. 2009;52(10):2037–45.
Brouhard BH, Lagrone L. Effect of dietary protein restriction on functional renal reserve in diabetic nephropathy. Am J Med. 1990;89(4):427–31.
Raal FJ, Kalk WJ, Lawson M, et al. Effect of moderate dietary protein restriction on the progression of overt diabetic nephropathy: a 6-mo prospective study. Am J Clin Nutr. 1994;60(4):579–85.
Hansen HP, Tauber-Lassen E, Jensen BR, Parving HH. Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int. 2002;62(1):220–8.
Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2007;(4):CD002181.
Waugh NR, Robertson AM. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2000;(2):CD002181.
Li XF, Xu J, Liu LJ, et al. Efficacy of low-protein diet in diabetic nephropathy: a meta-analysis of randomized controlled trials. Lipids Health Dis. 2019;18(1):82.
Kitada M, Ogura Y, Monno I, Koya D. A low-protein diet for diabetic kidney disease: its effect and molecular mechanism, an approach from animal studies. Nutrients. 2018;10(5):544.
Wrone EM, Carnethon MR, Palaniappan L, Fortmann SP, Third National Health and Nutrition Examination Survey. Association of dietary protein intake and microalbuminuria in healthy adults: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(3):580–7.
Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–84.
Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76(3S1):S1–107.
Kistler BM, Moore LW, Benner D, et al. The International Society of Renal Nutrition and Metabolism Commentary on the National Kidney Foundation and Academy of Nutrition and Dietetics KDOQI clinical practice guideline for nutrition in chronic kidney disease. J Ren Nutr. 2020;S1051–2276(20):30134–5.
Koppe L, Fouque D. The role for protein restriction in addition to renin-angiotensin-aldosterone system inhibitors in the management of CKD. Am J Kidney Dis. 2019;73(2):248–57.
Mitch WE, Walser M, Steinman TI, Hill S, Zeger S, Tungsanga K. The effect of a keto acid-amino acid supplement to a restricted diet on the progression of chronic renal failure. N Engl J Med. 1984;311(10):623–9.
Bellizzi V, Calella P, Hernandez JN, et al. Safety and effectiveness of low-protein diet supplemented with ketoacids in diabetic patients with chronic kidney disease. BMC Nephrol. 2018;19(1):110.
Koppe L, Cassani de Oliveira M, Fouque D. Ketoacid analogues supplementation in chronic kidney disease and future perspectives. Nutrients. 2019;11(9):2071.
Pedersen MMMC, Jorgensen FS, Moller B, Lykke G, Pedersen O. Renal effects from limitation of high dietary protein in normoalbuminuric diabetic patients. Kidney Int Suppl. 1989;27:S115–21.
Wiseman MJ, Bognetti E, Dodds R, Keen H, Viberti GC. Changes in renal function in response to protein restricted diet in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1987;30(3):154–9.
Levey AS, Greene T, Sarnak MJ, et al. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2006;48(6):879–88.
Stefikova K, Spustova V, Gazdikova K, Krivosikova Z, Dzurik R. Dietary protein restriction in combination with angiotensin converting enzyme inhibitor improves insulin resistance in patients with chronic renal disease. Int Urol Nephrol. 1997;29(4):497–507.
Wu HL, Sung JM, Kao MD, Wang MC, Tseng CC, Chen ST. Nonprotein calorie supplement improves adherence to low-protein diet and exerts beneficial responses on renal function in chronic kidney disease. J Ren Nutr. 2013;23(4):271–6.
Paes-Barreto JG, Silva MI, Qureshi AR, et al. Can renal nutrition education improve adherence to a low-protein diet in patients with stages 3 to 5 chronic kidney disease? J Ren Nutr. 2013;23(3):164–71.
Acknowledgements
Funding
This study and the Journal’s Rapid Service Fee were supported by the grants from National Natural Science Foundation (no. 81270816 and no. 81470974 to W.J.W) and High-level Hospital Construction Project of Guangdong Province (DFJH201908 to W.J.W).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributors
All authors significantly contributed to the work and approved the manuscript for submission. QL Li, YH Wang and F Wen contributed equally to the conception and designing of the study, all processes of the study methods and drafting of all sections of the manuscript. F Wen, YH Wang, JT Xie and WJ Wang designed the study’s analytic strategy and provided technical support in carrying out the statistical analyses. QL Li and F Wen selected studies that met the inclusion criteria and acquired the full papers of studies that should be left for further review. S Li, SC Lin, CF Qi, ZJ Chen, XQ Qiu, YF Zhang, SG Zhang, YM Tao, ZL Feng, ZL Li, RZ Li, ZM Ye, XL Liang and SX Liu gave various opinions in their interpretations of the study results and helped draft the manuscript. JT Xie and WJ Wang performed the study and revised the draft critically for important intellectual content.
Disclosures
Qiuling Li, Feng Wen, Yanhui Wang, Sheng Li, Shaochun Lin, Chunfang Qi, Zujiao Chen, Xueqian Qiu, Yifan Zhang, Shaogui Zhang, Yiming Tao, Zhonglin Feng, Zhilian Li, Ruizhao Li, Zhiming Ye, Xinling Liang, Shuangxin Liu, Jianteng Xie and Wenjian Wang have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Li, Q., Wen, F., Wang, Y. et al. Diabetic Kidney Disease Benefits from Intensive Low-Protein Diet: Updated Systematic Review and Meta-analysis. Diabetes Ther 12, 21–36 (2021). https://doi.org/10.1007/s13300-020-00952-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00952-5