Abstract
In recent years, the development of basal insulin therapies has focused on insulin analogues that have longer durations of action and more predictable pharmacokinetic/pharmacodynamic (PK/PD) profiles than their human insulin-based predecessors, such as neutral protamine Hagedorn (NPH) insulin. Dosed once-daily, such analogues can provide a more stable glucose-lowering action, which translates clinically into a reduced risk of hypoglycemia. Insulin degludec (degludec) became available in Canada in 2017 and is the first basal insulin analogue to have a half-life exceeding the dosing interval. As well as offering the promise of an exceptionally flat PK/PD profile when at steady state, this characteristic means that insulin degludec can be dosed with some flexibility with regard to time of day and that it need not be taken at the same time each day. However, the approximately 25-h half-life also has some implications concerning dose titration. This article provides an up-to-date review of the study data describing the clinical profile of degludec, and aims to give helpful and practical advice to prescribers about its use. While the clinical benefits of degludec are described, it is also acknowledged that further study is required to better understand how its clinical performance compares with that of insulin glargine 300 units/mL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Insulin degludec (degludec) is an innovative basal insulin with some unique pharmacological properties that translate into clinical benefits. |
Degludec carries a relatively low risk of hypoglycemia and can be dosed flexibly (at different times each day) to benefit patient convenience. |
The very long half-life of degludec means there are some important considerations that prescribers need to be mindful of regarding dose titration. |
Further studies are required to fully differentiate the clinical profiles of degludec and insulin glargine 300 units/mL. |
Digital Features
This article is published with digital features to facilitate understanding of the article. You can access the digital features on the article’s associated Figshare page. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12820778.
Introduction
Insulin degludec (degludec) was launched in Canada in late 2017 and is the first major new basal insulin product for 2 years (since the introduction of insulin glargine 300 units/mL [glargine U300]), and the first new basal insulin molecule for nearly 10 years (since insulin detemir). This article reviews the rationale for the clinical performance and utility of degludec in the light of the latest study data, and for its inclusion in the Canadian Clinical Practice Guidelines of April 2018. The need for some further comparative studies is also acknowledged.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
The Rationale for a New Generation Basal Insulin Analogue and the Limitations of Previously Available Basal Insulins
In normal human physiology, insulin is secreted so as to maintain a near-constant basal level in the circulation, upon which are superimposed rapidly produced and temporary elevations in response to food intake [1, 2]. Insulin replacement therapy in type 1 diabetes (T1D) attempts to recreate this profile through the use of basal and bolus insulin products, or subcutaneous pump infusion. In type 2 diabetes (T2D), where some pancreatic beta-cell function is preserved, insulin can be given as supplementary therapy, and is often started as basal insulin, which can be later intensified by adding bolus insulin or either a glucagon-like peptide-1 receptor agonist (GLP-1RA), dipeptidyl peptidase-4 inhibitor, or sodium-glucose linked transporter-2 inhibitor.
A more ideal basal insulin product would attempt to recreate the normal physiological profile arising from endogenous insulin output by accomplishing the following: (1) a near-constant (not fluctuating) plasma insulin level across 24 h, avoiding both peaks that could risk hypoglycemia or periods of low insulin concentration that could risk hyperglycemia; (2) a predictable glucose-lowering action from injection to injection, enabling patients to confidently adjust the dose in pursuit of their blood glucose targets; and (3) a low injection frequency requirement (≤ 1/day) for patient convenience and flexibility in dosing to accommodate individual lifestyles.
Basal insulin replacement has often been problematic, however, as the less than ideal pharmacokinetic (PK)/pharmacodynamic (PD) properties of the insulin products developed to date have made it difficult to mimic the body’s ability to adjust fasting insulin levels to physiological need using subcutaneous injection regimens. Neutral protamine Hagedorn (NPH) insulin was the standard basal insulin for many years, but it has a fluctuating and unpredictable PD profile [3], which may be even more unpredictable in clinical use due to patient failure to achieve adequate resuspension before injection [4]. NPH insulin also has a relatively short duration of glucose-lowering action, consistent with its half-life of approximately 4.4 h [5]. Unsurprisingly, therefore, NPH often requires twice-daily injection for full basal insulin replacement therapy, and its unpredictability carries a relatively greater risk of hypoglycemia than basal insulins developed subsequently [6].
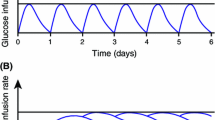
Insulin glargine 100 units/mL (glargine U100) represented an improvement, with clinical trials showing a reduced risk of hypoglycemia at equivalent glycated hemoglobin (A1C) when compared with NPH insulin [6]. For example, in insulin-naïve patients with T2D, statistically significant reductions in the rates of overall and nocturnal hypoglycemia in excess of 20 and 40%, respectively, were shown in the original treat-to-target (TTT) trial, where both insulins were titrated to a mean A1C level of < 7.0% [7]. However, glargine’s post-injection precipitation protraction mechanism was still associated with some unpredictability [3], and its duration of action was often shorter than anticipated, meaning that it was not reliable for use once daily for all patients (especially those with T1D) [6]. Glargine U100 has a half-life of approximately 12–13.5 h [8, 9]. This is a clear improvement on NPH insulin, but likely to result in a PK/PD profile with a relatively high peak:trough ratio when used once daily, which is not ideal for a basal insulin [10] (Fig. 1).
Hypothetical pharmacokinetic profiles of insulins with various half-lives, demonstrating that stacking does not occur when the half-life exceeds the dosing interval. Reproduced with permission from Heise and Meneghini [10]. a Accumulation from first dose to steady state. b Perturbations following various types of common dosing errors as indicated by arrows, when introduced at steady-state. Fluctuations in insulin concentration (and therefore glucose-lowering action) are greatest, and dosing errors have the most acute effects, with basal insulins having a short half-life (e.g., 6 h) and short duration of action. Fluctuations are dampened and dosing errors have less acute effects with basal insulins having a longer half-life/duration of action. The half-lives for basal insulin shown in the figure correspond approximately to those of neutral protamine Hagedorn, insulin glargine 100 units/mL, and insulin degludec, respectively (from left to right)
A more concentrated low-volume formulation of glargine has recently been released (glargine units/mL [glargine U300]), which not only permits smaller injection volumes, but modifies the PK/PD profile. Glargine U300 has a half-life of approximately 19 h [8] and hence has a longer duration of action than glargine U100. A meta-analysis of the non-blinded EDITION studies showed glargine U300 to lower the rate of nocturnal hypoglycemia by 18% compared with glargine U100 in patients with T2D (p < 0.05) [11]. In patients with T1D, one randomized, controlled, multinational trial found that hypoglycaemia did not differ for glargine U100 and glargine U300, with the exception that during the first 8 weeks of the study there was a significant 31% reduced hypoglycaemia in the glargine U300 treatment arm [12]. However, in a multicenter trial of Japanese T1D patients, the annualized rates of confirmed or severe hypoglycemia were 34% lower with glargine U300 at night and 20% lower overall (statistically significant for both) [13]. In a pilot study of 18 young T1D patients with poor glycemic control on morning glargine U100 (mean A1C 7.45% after optimizing prior therapy for 12 weeks) and having hypoglycemic episodes (mean of 0.28 nocturnal events during 12-week optimization period), a switch to glargine U300 resulted in improved A1C (6.90%) and reduced hypoglycemia risk 6 months after the switch [14]. However, the unit-to-unit doses are not equivalent for glargine U100 and U300, and upward dose adjustments are generally required when patients are switched to glargine U300 [15].
Insulin detemir uses reversible albumin binding as a protraction mechanism [16] and this also buffers against variable absorption [17], resulting in a more predictable glucose-lowering action profile [3] and, again, a reduced risk of hypoglycemia compared with NPH insulin [6]. However, it has a half-life of 5–7 h [18] and, therefore, a suboptimal duration of action. This means it may require dosing twice daily, and its relative unit potency compared with glargine U100 can be low, especially in patients with T2D [19, 20]. As a result, insulin detemir has gained little market penetration in Canada.
In summary, glargine (U100 and U300) and insulin detemir represent an improvement from NPH insulin, with less risk of hypoglycemia (especially for nocturnal episodes) as demonstrated in comparative trials. This risk reduction has also been shown to translate into a decreased rate of hospitalization for severe hypoglycemia and secondary healthcare visits in real-world patients (21.7% lower [p < 0.001] for detemir compared with NPH insulin and 9.9% lower [p = 0.022] for glargine U100 compared with NPH) [21].
However, there has remained an on-going need for an improved basal insulin that can be reliably dosed once daily to provide a glucose-lowering effect that is stable across 24 h, and predictable from day-to-day. Such an insulin should, in theory, allow fasting glucose targets to be more easily met with a lower risk of hypoglycemia.
The Unique Mechanism of Protraction and PK/PD Profile of Degludec
Structurally, degludec has similarities to insulin detemir in that it is acylated with a side chain attached to the B29 amino acid. However, the side chain of degludec differs from that of insulin detemir in being a hydrophilic fatty diacid linked via a spacer [22]. This molecular structure allows degludec to bind reversibly to albumin (as per insulin detemir, and hence sharing the benefit of being able to buffer absorption rate changes), but it also results in the self-association state of degludec changing from dihexamer to multihexamer chains after injection. These chains subsequently release monomers at a slow and steady rate, which is the primary mechanism of protraction [22]. Another spin-off benefit of the molecular structure of degludec is that it can be co-formulated with other insulins and GLP-1RAs. Co-formulations of previously available insulins was impossible as a result of chemical incompatibilities or the formation of hybrid insulin hexamers with unpredictable absorption kinetics [23]. Consequently, degludec has been developed as two co-formulation products, one with the rapid-acting insulin aspart (IDegAsp), and one with the GLP-1RA, liraglutide (IDegLira—approved for use in Canada).
With a half-life of > 25 h, degludec is the only basal insulin that has a half-life that exceeds the dosing interval [9]. Although it is common for pharmaceuticals to be dosed at intervals that are shorter than their half-lives, some prescribers who are familiar with the concept of insulin stacking might fear accumulation. In fact, this does not occur with basal insulin therapy; rather, the PK effect is merely to lengthen the time needed to reach a steady-state, which is then characterized by a very low peak:trough ratio [10] (Fig. 1). This property also means that dose intervals can be variable without unduly affecting the risk of hyper/hypoglycemia [10, 24,25,26]. Thus, once-daily degludec produces a near-constant flat and stable glucose-lowering action over 24 hours [27], with relatively little variability in this effect from injection-to-injection in comparison with insulin glargine U100 (total metabolic effect: area under the glucose infusion rate graph at steady state between 0 and 24 h, coefficient of variation of 20% for degludec compared with 82% for insulin glargine U100) [28].
On the other hand, the protracted time to steady state means that dose adjustments have to be made (and the effect assessed) at greater intervals compared with other basal insulins, as elaborated in following sections. Furthermore, the more constant blood glucose-lowering effect of degludec could potentially impact the optimal dosing of bolus insulin in patients on multiple injection therapy. A 200 units/mL (U200) formulation of degludec is also available that allows high doses to be given in reduced injection volumes. Importantly, and in contrast to the case with glargine U100 and U300, degludec U200 has bioequivalence to degludec U100 [29], meaning that unit-for-unit transfers between the products are possible, with the two formulations having similar clinical profiles [30].
The Clinical Profile of Degludec: What Did We Learn from the Phase 3 Trial Program?
The phase 3 trial program compared degludec almost exclusively with glargine U100 in a variety of cohorts: T1D [25, 31,32,33], insulin-naïve T2D [26, 34,35,36], and insulin-experienced T2D [26, 37, 38]. The studies had TTT designs and used more aggressive titration algorithms (mostly targeting fasting glucose of 3.9 to < 5.0 mmol/L) compared with previous TTT trials (typically targeting ≤ 5.5 mmol/L) and the EDITION studies (4.4–5.6 mmol/L). At equivalent A1C levels, degludec was generally associated with lower risks for hypoglycemia—particularly for nocturnal events, which are more influenced by the basal component of a basal–bolus regimen. Pooled patient-level data for self-reported hypoglycemia from seven phase 3 trials have been analyzed by Ratner [39]. Among insulin-naïve patients with T2D, those using degludec had statistically significantly lower rates of overall confirmed, nocturnal confirmed, and severe hypoglycemic episodes than those using glargine U100, with estimated rate ratios (RRs) of 0.83, 0.64, and 0.14, respectively. In the overall T2D population, patients using degludec also had significantly lower rates of overall confirmed and nocturnal confirmed episodes compared with those using glargine U100, with respective RR values of 0.83 and 0.68. For patients with T1D, the rate of nocturnal confirmed episodes was significantly lower with degludec compared with glargine U100 during maintenance treatment (RR 0.75).
Subsequent meta-analyses have supported the conclusion of a reduced risk of nocturnal hypoglycemia in various subgroups (the elderly [40]; patients with T2D receiving a high dose [35]; T1D patients achieving good control [41]) and using different study data [42, 43]. A summary of the results of these meta-analyses is presented in Table 1. The lower rate of nocturnal hypoglycemia with degludec compared with glargine U100 in patients with T1D and T2D was further confirmed by another meta-analysis using three different definitions of nocturnal hypoglycemia and different timescales for the nocturnal period [44].
Two 26-week, randomized, TTT trials, one in T1D [25] and one in T2D [26], demonstrated the versatility of degludec when it comes to the dosing interval, offering patients the prospect of freedom from very strict dose times. In the T1D trial, degludec administered in a ‘Force-Flex’ schedule using rather extreme varied dosing intervals (alternating intervals of a minimum of 8 h and maximum of 40 h between doses) was compared with degludec or glargine U100 given at the same time each day (Free-Flex regimen) [25]. At 26 weeks, A1C had decreased in all treatment arms, and the Force-Flex regimen met the noninferiority criteria compared with both Free-Flex regimens. The rates of confirmed hypoglycemia were similar in all treatment arms at weeks 26 and 52, but nocturnal confirmed hypoglycemia was lower with the degludec Forced-Flex regimen than with the degludec Free-Flex regimen (37% lower; p = 0.003) and the glargine U100 Free-Flex regimen (40% lower; p = 0.001). In the T2D trial, using the following dosing schedule, namely, degludec Force-Flex regimen, degludec given once-daily in the evening (degludec OD), and glargine U100 given at the same time each day (glargine OD), A1C improved for all three dosing regimens at 26 weeks, and noninferiority was also demonstrated for the Forced-Flex regimen compared with glargine OD [26]. There were also no statistically significant differences in overall or nocturnal hypoglycemia rates between the degludec Force-Flex regimen and the other regimens. Similar levels of glycemic control and hypoglycemia rates were also obtained in a Japanese study that compared degludec at a fixed dose time of the patient’s choosing with degludec administered flexibly, but within 8 h of an agreed dose time [24].
The Clinical Profile of Degludec: What Have We Learned from More Recent Studies?
To further assess the relative risk of hypoglycemia with degludec, the US Food and Drug Administration recommended two blinded crossover trials—the SWITCH studies, which included at-risk patients who would have been excluded from traditional insulin trials [45, 46]. In these TTT studies, patients were initially randomized to either degludec or glargine U100 treatment arms, and then at 32 weeks switched to the opposite treatment arm. During the full treatment period, T1D patients using degludec had a significantly lower rate of overall symptomatic hypoglycemia compared with those using glargine U100 (RR 0.94, p = 0.002) and a significantly lower rate of nocturnal symptomatic hypoglycemia (RR 0.75, p < 0.001). In the T2D SWITCH study, the respective estimated rate ratios were 0.77 (p < 0.001) and 0.75 (p < 0.001), again favoring degludec. The rate of severe hypoglycemia was significantly lower for degludec over the full treatment period for patients with T1D (RR 0.74, p = 0.003) and T2D (RR 0.49, p = 0.03).
Meanwhile, the large-scale, double-blinded, DEVOTE cardiovascular outcomes trial (CVOT) was conducted (at 438 sites in 20 countries) in > 7600 patients with T2D who were at high cardiovascular risk (85.2% had established cardiovascular disease, chronic kidney disease, or both). Patients were randomized to either the degludec or glargine U100 treatment arms (1:1) and followed for 24 months; the primary outcome was first occurrence of an adjudicated major cardiovascular event (MACE). Adjudicated severe hypoglycemia was a pre-specified secondary outcome. This trial generated a vast database that has led to a number of important discoveries: (1) degludec was shown not to elevate MACE risk compared with glargine U100 (hazard ratio 0.91, 95% confidence interval [CI] 0.78–1.06, p < 0.001 for noninferiority) and a significantly lower relative risk for severe hypoglycemic events with degludec was confirmed (RR 0.60, p < 0.001) [47]; (2) this risk reduction drove the finding, in a health economic analysis, that degludec compared with glargine U100 was at least cost neutral over a 2-year timeframe in a UK setting [48]; (3) an association between all-cause mortality and hypoglycemia (previously observed in other studies) was confirmed [49, 50]; (4) a known association between glucose variability and hypoglycemia was likewise also confirmed [50].
More recently, some comparisons with glargine U300 have been undertaken. Clamp studies have shown degludec to have a theoretically superior PD profile [51], but available clinical studies give inconsistent results. For example, in the first head-to-head trial (BRIGHT study), which was conducted in insulin-naïve (and hence low hypoglycemia risk) patients, there were no significant differences in the relative risks for overall confirmed or nocturnal confirmed hypoglycemia with glargine U300 compared with degludec over the 24-week study period [52]. There were, however, some risk reductions favoring glargine U300 in the first 12 weeks of the study (e.g., RRs of 0.77 [95% CI 0.62–0.96] and 0.65 [95% CI 0.43–0.98], respectively, for overall and nocturnal confirmed hypoglycemia with blood glucose ≤ 70 mg/dL [3.9 mmol/L]), but equipotent doses of the two insulins were likely not being administered during this time (based on the end-of-trial doses), and no significant differences in hypoglycemia rates were observed in weeks 13–24. There was no statistically significant difference in end-of-trial glycemic control, but the daily insulin dose was 20% lower in patients on degludec than in those receiving glargine U300 (0.43 vs. 0.54 U/kg).
In contrast, a real-world-evidence study (CONFIRM study) used propensity scoring to compare clinical outcomes with these two insulins for > 4000 insulin-naïve patients in the USA [53]. This study found statistically significant advantages for degludec over glargine U300 in terms of A1C reduction (estimated treatment difference − 0.3% [p < 0.05]), a 30% lower risk of hypoglycemia (RR 0.70, p < 0.05), and a statistically significant 27% lower likelihood of treatment discontinuation. It is, however, unwise to make direct comparisons between prospective randomized controlled studies (such as BRIGHT, with its target-driven titration algorithms) and retrospective real-world evidence studies since the conditions of use and hence the clinical questions posed are so different. The results of further head-to-head studies are awaited, and differences in dose potency need to be accounted for as this appears to be a confounding factor.
Using Degludec to Optimum Effect: Prescribing Recommendations and Practical Tips
The fear of hypoglycemia and a lack of confidence about dose titration are important barriers to the initiation and intensification of insulin therapy, especially in patients with T2D [54]. In patients with T1D, insulin therapy is usually established and guided under specialist care, but insulin therapy for patients with T2D, particularly the initiation of basal insulin, is encouraged as a primary care intervention. The burden of T2D is high and increasing, and many patients languish in a state of poor glycemic control, putting them at risk of complications. For example, in the multinational SOLVE study, the mean A1C of people with T2D initiating insulin therapy was 8.9 ± 1.6%, with 40.9% of subjects having A1C ≥ 9.0% (Canadian numbers were very similar) [55]. Primary healthcare providers and pharmacists (who tend to have the most contact with T2D patients) are therefore encouraged to recognize the need for, and to effect, the initiation and titration of insulin. Furthermore, the absolute risk of hypoglycemia in T2D is much lower than in T1D, even among patients using insulin. For example, in one study of insulin users, there were 16.4 and 42.9 events per person-year, in the patient populations with T2D and T1D, respectively [56]. This lower risk may be explained in part by the reduced insulin sensitivity of patients with T2D and the fact that insulin use is often supplementary. In the context of basal–bolus therapy, most hypoglycemia results from a mismatch between mealtime insulin dosing and carbohydrate consumption, although nocturnal hypoglycemia is more likely to be due to inappropriate basal insulin kinetics (as well as the use of some oral hypoglycemic agents by patients with T2D).
Although degludec has some unique PK/PD properties that require special consideration, it has optimal properties for use in primary care in T2D as a result of its low hypoglycemia risk and dose-time flexibility. The very constant glucose-lowering effect of degludec over 24 h may be especially beneficial given the latest Diabetes Canada treatment guidelines, which encourage the targeting of low A1C values, achieved through more aggressive lowering of fasting glucose values when needed, as well as postprandial glucose control:
Relevant recommendations from Diabetes Canada 2018 guidelines [66]
Glucose targets In most people with T1D or T2D, an A1C measurement of ≤ 7.0% should be targeted to reduce the risk of microvascular, and, if implemented early in the course of disease, cardiovascular complications In people with T2D, an A1C measurement of ≤ 6.5% may be targeted to reduce the risk of chronic kidney disease and retinopathy, if they are assessed to be at low risk of hypoglycemia A higher A1C target may be considered in people with diabetes with the goals of avoiding hypoglycemia and over-treatment related to antihyperglycemic therapy, with any of the following: • Functionally dependent: 7.1–8.0% • History of recurrent severe hypoglycemia, especially if accompanied by hypoglycemia unawareness: 7.1–8.5% • Limited life expectancy: 7.1–8.5% • Frail elderly and/or with dementia: 7.1–8.5% • End of life: A1C measurement not recommended. Avoid symptomatic hyperglycemia and any hypoglycemia In order to achieve an A1C measurement of A1C ≤ 7.0%, people with diabetes should aim for: • Fast plasma glucose (FPG) or preprandial plasma glucose target of 4.0–7.0 mmol/L and a 2-h postprandial glucose (PPG) target of 5.0–10.0 mmol/L • If an A1C target of ≤ 7.0% cannot be achieved with a FPG target of 4.0–7.0 mmol/L and PPG target of 5.0–10.0 mmol/L, further FPG lowering to 4.0–5.5 mmol/L and/or PPG lowering to 5.0–8.0 mmol/L may be considered, but must be balanced against the risk of hypoglycemia |
Insulin use in T1D In adults with T1D on basal-bolus injection therapy: • A long-acting insulin analogue may be used in place of NPH to reduce the risk of hypoglycemia, including nocturnal hypoglycemia • Degludec may be used instead of detemir or glargine U100 to reduce nocturnal hypoglycemia All individuals with T1D and their support persons should be counselled about the risk and prevention of hypoglycemia, and risk factors for severe hypoglycemia should be identified and addressed |
Insulin use in T2D For adults with T2D with metabolic decompensation (e.g. marked hyperglycemia, ketosis or unintentional weight loss), insulin should be used Insulin may be used at any time in the course of T2D. In people not achieving glycemic targets on existing noninsulin antihyperglycemic medication, the addition of a once-daily basal insulin regimen should be considered over premixed insulin or bolus only regimens, if lower risk of hypoglycemia and/or weight gain are priorities In adults with T2D treated with basal insulin therapy, if lower risk of hypoglycemia is a priority: • Long-acting insulin analogues (glargine U100, glargine U300, detemir, degludec) should be considered over NPH to reduce the risk of nocturnal and symptomatic hypoglycemia • Degludec may be considered over glargine U100 to reduce overall and nocturnal hypoglycemia • [Glargine U300 can also be considered for the same reasons] All individuals with T2D currently using or starting therapy with insulin or insulin secretagogues should be counselled about the prevention, recognition and treatment of hypoglycemia |
In many respects, the use of degludec is similar to that of any other basal insulin, but prescribers must always be mindful of its very long half-life and hence the longer time required to reach steady state and assess the impact of any dose adjustments. A drug dose will reach 90% of its steady-state level after three half-lives, equating to approximately 3 days for degludec [10]; consequently, the recommended interval between dose increases is 3–4 days [57, 58]. In practice, however, our empirical view is that some patients might even find it more convenient to titrate degludec weekly based on once-weekly fasting glucose testing.
Most practical considerations concerning the injection, dosing, storage, and handling of degludec are covered clearly in the US and Canadian degludec prescribing information [58, 59]. We also refer readers to a useful and detailed elaboration of good practice with insulin injection, including Canadian guidelines, which is freely available online from The Forum for Injection Technique (www.fit4diabetes.com). A few further practical suggestions based on our clinical experience are outlined below, but we stress the empirical nature of this content.
Firstly, the very stable PD profile of degludec at steady state, as well as reducing the risk of hypoglycemia also means that the timing of the daily dose is not critical, aiding convenience and simplicity. Hence, degludec can be injected once daily at any time of day, and this need not be the same time each day in adults, as long as at least 8 h have elapsed since the last injection. It is the authors’ opinion that it can be helpful to start degludec with morning dosing, if convenient. This is often more practical for the patient as they will be titrating the dose according to fasting glucose levels, and hence may find the process less confusing than titrating a night-time insulin dose based on prior fasting glucose values. Again, the long and near-constant glucose-lowering action of degludec facilitates the ability to both dose in the morning and titrate against fasting glucose values. Evening dosing is often recommended for other basal insulins because these insulins will be close to peak plasma levels (Cmax) at the time fasting glucose is measured. Earlier dosing of these insulins would bring the time of Cmax (tmax) into the night-time period, hence titration to the same fasting glucose target could risk nocturnal hypoglycemia. Consequently, many patients are obliged to take a late evening or bedtime dose (or to divide their basal dose between morning and evening injections), but this is seldom the case with degludec.
Prescribers (and patients) can be reassured that if a dose is inadvertently missed, then this can be taken in the waking hours upon discovery, again insuring that at least 8 h elapse before the next dose is taken. However, a single missed dose will be less consequential than with other basal insulins, as it will not result in a total loss of basal insulin coverage, which could result in perturbed control in T1D and advanced T2D.
While the product label stresses the need for individualized titration, the authors have found that T2D patients can often successfully manage their dosing by using a simple once-weekly self-titration schedule originally tested by Philis-Tsimikas et al. [60] (Table 2). Using this simple titration algorithm (in which the dose is either kept the same or increased/decreased by 4 units according to a single pre-breakfast blood glucose value), A1C reduction was non-inferior to a more elaborate ‘step-wise’ titration algorithm, and there were no significant differences in other control and tolerability outcomes. As an alternative, a simple twice-weekly titration algorithm has also been proposed [61]. In this algorithm, 2 titration days are nominated at 3- to 4-day intervals; the degludec dose is left unchanged if the fasting glucose on the titration day is within the range of 4.0 and 7.0 mmol/L, but decreased or increased by 2 units if the value is < 4.0 or > 7.0 mmol/L, respectively; doses are then maintained until the next titration day.
Transferring to degludec from another basal insulin is straightforward if the Canadian prescribing information recommendations are followed [59]. In patients with T1D, degludec is started with a 20% reduction in the number of previous total daily basal insulin units. Patients with T2D with good glycemic control previously taking a basal insulin once daily can be switched to degludec unit for unit; however, for those patients with T2D previously taking their basal insulin twice daily or those previously using once-daily glargine U300, the unit starting dose for degludec should again be reduced by 20%.
Degludec is available as Tresiba® (Novo Nordisk, Bagsværd, Denmark) in the disposable prefilled FlexTouch® pen (Novo Nordisk), with instructions for use provided in the prescribing information. The dose to be injected is selected by twisting a dose-selector dial with the number of units visible in a display window. The dialing mechanism also produces audible clicks as the number of units selected changes. The FlexTouch pen requires a low pressure to effect an injection [62], has good dosing accuracy [63], and was preferred to SoloSTAR® (Sanofi S.A., Paris, France) by doctors, nurses, and diabetes patients in a comparative study [64].
Two formulations of Tresiba are available. The Tresiba U100 FlexTouch pen contains 300 units of degludec. It delivers doses in 1-unit increments and can deliver up to 80 units in a single injection. The Tresiba U200 FlexTouch pen contains 600 units of degludec and delivers doses in 2-unit increments, up to 160 units in a single injection. No dose conversion is required when switching between these formulations, and the dose window of each pen shows the number of insulin units to be delivered.
If a hypoglycemic episode occurs, it should be treated by usual means; the long action of degludec should not be a cause for concern because the acute glucose-lowering effect will be proportional to the plasma level and hence will be no more intense than with any other basal insulin. Indeed, the physiological response to, and recovery time from, induced hypoglycemia in insulin-treated T1D patients have been shown to be similar comparing degludec with glargine U100 [65].
Conclusion
Degludec achieves a very long and stable glucose-lowering profile through a unique protraction mechanism. This translates into a low risk of hypoglycemia, the ability to dose in the morning or flexibility, and a ‘forgiving’ profile should doses be missed or mis-timed. The scale of the hypoglycemia advantage with the newer analogues, degludec and glargine U300, is comparable to that demonstrated for glargine U100 and insulin detemir over NPH some 15 years ago, when standard of care shifted away from NPH. The relative clinical advantages/disadvantages of degludec compared with glargine U300 require clarification and are currently under further investigation.
References
Kruszynska YT, Home PD, Hanning I, Alberti KG. Basal and 24-h C-peptide and insulin secretion rate in normal man. Diabetologia. 1987;30:16–211.
Polonsky KS, Given BD, Van Cauter E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest. 1988;81:442–8.
Heise T, Nosek L, Ronn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–20.
Vora J, Heise T. Variability of glucose-lowering effect as a limiting factor in optimizing basal insulin therapy: a review. Diabetes Obes Metab. 2013;15:701–12.
Lilly USA LLC. Humulin H—highlights of the prescribing information. 2019. https://pi.lilly.com/us/humulin-r-pi.pdf.
DeVries JH, Nattrass M, Pieber TR. Refining basal insulin therapy: what have we learned in the age of analogues? Diabetes Metab Res Rev. 2007;23:441–54.
Riddle MC, Yki-Jarvinen H, Bolli GB, et al. One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the EDITION 1 12-month randomized trial, including 6-month extension. Diabetes Obes Metab. 2015;17:835–42.
Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL−1. Diabetes Care. 2015;38:637–43.
Heise T, Hovelmann U, Nosek L, Hermanski L, Bottcher SG, Haahr H. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert Opin Drug Metab Toxicol. 2015;11:1193–201.
Heise T, Meneghini LF. Insulin stacking versus therapeutic accumulation: understanding the differences. Endocr Pract. 2014;20:75–83.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Jarvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:541–8.
Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 Units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care. 2015;38:2217–25.
Matsuhisa M, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016;18:375–83.
Gradiser M, Berkovic MC, Bilic-Curcic I. Changes in HbA1c and hypoglycemic episodes in type 1 diabetes patients after switching to insulin glargine U300: pilot study. Diabetes Res Clin Pract. 2017;129:144–7.
Pearson SM, Trujillo JM. Conversion from insulin glargine U-100 to insulin glargine U-300 or insulin degludec and the impact on dosage requirements. Ther Adv Endocrinol Metab. 2018;9:113–21.
Havelund S, Plum A, Ribel U, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;21:1498–504.
Kurtzhals P. How to achieve a predictable basal insulin? Diabetes Metab. 2005;31:4S25–4S33.
Novo Nordisk. Levemir® Prescribing Information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021536s054lbl.pdf. Accessed Feb 2019.
Bryant GA, McDanel DL, Horner KE, Farris KB, Newkirk EN. Evaluation of dosing and clinical outcomes in patients undergoing conversion of insulin glargine to insulin detemir. Pharmacotherapy. 2013;33:56–62.
Wallace JP, Wallace JL, McFarland MS. Comparing dosing of basal insulin analogues detemir and glargine: is it really unit-per-unit and dose-per-dose? Ann Pharmacother. 2014;48:361–8.
Haukka J, Hoti F, Erasto P, Saukkonen T, Makimattila S, Korhonen P. Evaluation of the incidence and risk of hypoglycemic coma associated with selection of basal insulin in the treatment of diabetes: a Finnish register linkage study. Pharmacoepidemiol Drug Saf. 2013;22:1326–35.
Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29:2104–14.
Havelund S, Ribel U, Hubalek F, Hoeg-Jensen T, Wahlund PO, Jonassen I. Investigation of the physico-chemical properties that enable co-formulation of basal insulin degludec with fast-acting insulin aspart. Pharm Res. 2015;32:2250–8.
Kadowaki T, Jinnouchi H, Kaku K, Herslov ML, Hyllested-Winge J, Nakamura S. Efficacy and safety of once-daily insulin degludec dosed flexibly at convenient times vs fixed dosing at the same time each day in a Japanese cohort with type 2 diabetes: a randomized, 26-week, treat-to-target trial. J Diabetes Investig. 2016;7:711–7.
Mathieu C, Hollander P, Miranda-Palma B, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013;98:1154–62.
Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36:858–64.
Heise T, Nosek L, Bottcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–50.
Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–64.
Korsatko S, Deller S, Koehler G, et al. A comparison of the steady-state pharmacokinetic and pharmacodynamic profiles of 100 and 200 U/mL formulations of ultra-long-acting insulin degludec. Clin Drug Investig. 2013;33:515–21.
Bode BW, Chaykin LB, Sussman AM, et al. Efficacy and safety of insulin degludec 200 U/mL and insulin degludec 100 U/mL in patients with type 2 diabetes (Begin: Compare). Endocr Pract. 2014;20:785–91.
Davies MJ, Gross JL, Ono Y, et al. Efficacy and safety of insulin degludec given as part of basal-bolus treatment with mealtime insulin aspart in type 1 diabetes: a 26-week randomized, open-label, treat-to-target non-inferiority trial. Diabetes Obes Metab. 2014;16:922–30.
Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1489–97.
Bode BW, Buse JB, Fisher M, et al. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal-bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN((R)) Basal-Bolus Type 1): 2-year results of a randomized clinical trial. Diabet Med. 2013;30:1293–7.
Gough SCL, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal R. Low volume insulin degludec 200 units/mL once daily improves glycemic control similar to insulin glargine with a low risk of hypoglycemia in insulin-naïve patients with type 2 diabetes: a 26-week, randomized, controlled, multinational, treat-to-target trial: the BEGIN™ LOW VOLUME trial. Diabetes Care. 2013;36:2536–42.
Rodbard HW, Cariou B, Zinman B, et al. Comparison of insulin degludec with insulin glargine in insulin-naive subjects with type 2 diabetes: a 2-year randomized, treat-to-target trial. Diabet Med. 2013;30:1298–304.
Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35:2464–71.
Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498–507.
Hollander P, King AB, Del Prato S, et al. Insulin degludec improves long-term glycaemic control similarly to insulin glargine but with fewer hypoglycaemic episodes in patients with advanced type 2 diabetes on basal-bolus insulin therapy. Diabetes Obes Metab. 2015;17:202–6.
Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175–84.
Sorli C, Warren M, Oyer D, Mersebach H, Johansen T, Gough SC. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009–188.
Einhorn D, Handelsman Y, Bode BW, Endahl LA, Mersebach H, King AB. Patients achieving good glycemic control (HbA1c <7%) experience a lower rate of hypoglycemia with insulin degludec than with insulin glargine: a meta-analysis of phase 3a trials. Endocr Pract. 2015;21:917–26.
Dzygalo K, Golicki D, Kowalska A, Szypowska A. The beneficial effect of insulin degludec on nocturnal hypoglycaemia and insulin dose in type 1 diabetic patients: a systematic review and meta-analysis of randomised trials. Acta Diabetol. 2015;52:231–8.
Russell-Jones D, Gall MA, Niemeyer M, Diamant M, Del Prato S. Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: a meta-analysis of seven clinical trials. Nutr Metab Cardiovasc Dis. 2015;25:898–905.
Heller S, Mathieu C, Kapur R, Wolden ML, Zinman B. A meta-analysis of rate ratios for nocturnal confirmed hypoglycaemia with insulin degludec vs. insulin glargine using different definitions for hypoglycaemia. Diabet Med. 2016;33:478–87.
Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs uinsulin glargine U100 on hjypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA. 2017;318:33–44.
Wysham C, Bhargava A, Chaykin L, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes: the SWITCH 2 randomized clinical trial. JAMA. 2017;318:45–56.
Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–32.
Pollock RF, Valentine WJ, Marso SP, et al. DEVOTE 5: evaluating the short-term cost-utility of insulin degludec versus insulin glargine U100 in basal-bolus regimens for type 2 diabetes in the UK. Diabetes Ther. 2018;9:1217–32.
Pieber TR, Marso SP, McGuire DK, et al. DEVOTE 3: temporal relationships between severe hypoglycaemia, cardiovascular outcomes and mortality. Diabetologia. 2018;61:58–655.
Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia. 2018;61:48–57.
Heise T, Norskov M, Nosek L, Kaplan K, Famulla S, Haahr HL. Insulin degludec: lower day-to-day and within-day variability in pharmacodynamic response compared with insulin glargine 300 U/mL in type 1 diabetes. Diabetes Obes Metab. 2017;19:1032–9.
Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus insulin degludec 100 units/mL in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41:2147–54.
Tibaldi J, Hadley-Brown M, Liebl A, et al. A comparative effectiveness study of degludec and insulin glargine 300 U/mL in insulin-naive patients with type 2 diabetes. Diabetes Obes Metab. 2019;21:1001–9.
Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20:488–96.
Khunti K, Damci T, Meneghini L, Pan CY, Yale JF. Study of once daily levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14:654–61.
Donnelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in type 1 and insulin-treated type 2 diabetes: a population-based study. Diabet Med. 2005;22:749–55.
Appendix 9—examples of insulin initiation and titration regimens in people with type 2 diabetes. Can J Diabetes. 2018;42:S317-S8.
Novo Nordisk A/S. Tresiba® prescribing information. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203314s010lbl.pdf. Accessed Feb 2019.
Novo Nordisk Canada Inc. Tresiba product monograph. 2019. https://www.novonordisk.ca/content/dam/Canada/AFFILIATE/www-novonordisk-ca/OurProducts/PDF/tresiba-product-monograph.pdf. Accessed Sept 2020.
Philis-Tsimikas A, Brod M, Niemeyer M, Ocampo Francisco AM, Rothman J. Insulin degludec once-daily in type 2 diabetes: simple or step-wise titration (BEGIN: once simple use). Adv Ther. 2013;30:607–22.
Berard L, MacNeill G. Insulin degludec, a long-acting once-daily basal analogue for type 1 and type 2 diabetes mellitus. Can J Diabetes. 2015;39:4–9.
Gudiksen N, Hofstatter T, Ronn BB, Sparre T. FlexTouch: an insulin pen-injector with a low activation force across different insulin formulations, needle technologies, and temperature conditions. Diabetes Technol Ther. 2017;19:603–7.
Abdel-Tawab M, Schmitz M, Kamlot S, Schubert-Zsilavecz M. Dosing accuracy of two disposable insulin pens according to new ISO 11608–1: 2012 requirements. J Diabetes Sci Technol. 2015;10:157–61.
Pfutzner A, Forst T, Niemeyer M, Bailey T. Assessment for ease of use and preference of a new prefilled insulin pen (FlexTouch Degludec U100/U200) versus the SoloSTAR insulin pen by patients with diabetes and healthcare professionals. Expert Opin Drug Deliv. 2014;11:1381–9.
Köehler G, Heller S, Korsatko S, et al. Insulin degludec is not associated with a delayed or diminished response to hypoglycaemia compared with insulin glargine in type 1 diabetes: a double-blind randomised crossover study. Diabetologia. 2014;57:40–9.
Imran SA, Agarwal G, Bajaj HS, Ross S. Targets for glycemic control. Can J Diabetes. 2018;42:S42–S4646.
Acknowledgements
The authors would like to thank Dr Harpreet Bajaj, who contributed to an early draft of the manuscript and Jina Hahn (Novo Nordisk) for providing a Medical Accuracy Review of the outline and final draft.
Funding
Medical writing and submission support including the journals rapid service fee was provided by Murray Edmunds and Germanicus Hansa-Wilkinson of Watermeadow Medical, an Ashfield company, funded by Novo Nordisk.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Vincent Woo declares personal fees from Novo Nordisk during the conduct of the study and personal fees from Sanofi and Lilly outside the submitted work. Lori Berard has received consulting and/or is a member of the speaker bureau for Novo Nordisk, Eli Lilly, Sanofi, and Mylan. Robert Roscoe is a member of the speaker bureau/advisory board at, and reports personal fees from, Novo Nordisk, Abbott Diabetes Care, Janssen Pharmaceuticals, AstraZeneca, Merck Canada, Sanofi Canada, Becton Dickinson Canada, MontMed, and Eli Lilly. He is also a member of the speaker bureau at Bayer Healthcare Canada, Medtronic Canada, and Boehringer Ingelheim.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the compilation of this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12820778.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Woo, V., Berard, L. & Roscoe, R. Understanding the Clinical Profile of Insulin Degludec, the Latest Basal Insulin Approved for Use in Canada: a Narrative Review. Diabetes Ther 11, 2539–2553 (2020). https://doi.org/10.1007/s13300-020-00915-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00915-w