Abstract
The novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) outbreak originating in December 2019 has resulted in a worldwide pandemic affecting millions across almost 200 countries. People with diabetes appear to develop more severe forms of the disease and to require intensive care unit support and/or mechanical ventilation more frequently than those with other underlying medical conditions. The mortality rate among people with diabetes is also significantly higher than that among people without diabetes. A diagnosis of diabetes is often an indicator of poor underlying metabolic health, and frequently people with diabetes have multiple risk factors for severe coronavirus disease 2019 (COVID-19), including cardiovascular and renal disease. In this review, we discuss the potential biological mechanisms by which SARS-CoV-2 may interact with disease processes implicated in diabetes and discuss how treatments commonly used for people with diabetes may affect COVID-19 severity and progression. There is currently a lack of evidence from human studies, and further trials in this area will prove useful to further expand our understanding of this rapidly developing disease process to improve outcomes for this high-risk group of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A complex interaction of risk factors exist which are associated with both diabetes and increased mortality from coronavirus disease 2019 (COVID-19). |
Several underlying biological pathways exist which may explain the increased risk of mortality in people with diabetes from COVID-19. |
Medications used for people with diabetes appear to interact with some of the mechanisms associated with increased mortality, and further investigation is required to determine the clinical impact of these drugs. |
Diabetes and Novel Coronavirus Infection: Implications for Treatment video (MP4 1633936 kb)
Background
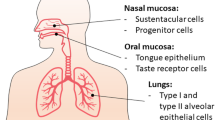
Coronaviruses have been implicated as a cause of respiratory and gastrointestinal infections since the isolation of the severe acute respiratory syndrome coronavirus (SARS-CoV-1) in Guangdong, China in 2002 [1] and Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia in 2012 [2]. Coronaviruses are enveloped, positive single-stranded RNA viruses which originated in bats. Indeed, both SARS-CoV-1 and MERS-CoV were transmitted initially to humans from market civets and dromedary camels, respectively [3]. In humans, SARS-CoV-1 infects ciliated bronchial epithelial cells and type II pneumocytes using angiotensin-converting enzyme 2 (ACE2) as a receptor, and MERS-CoV utilises the dipeptidyl peptidase-4 (DPP-4) receptor to infect both unciliated bronchial epithelial cells and type II pneumocytes [3].
More recently, a novel coronavirus, subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was isolated in Wuhan, China in December 2019 which causes the disease termed coronavirus disease 2019 (COVID-19) [4]. Subsequently, a worldwide pandemic has resulted, with over 5.5 million people infected and almost 350,000 deaths in 188 countries at the time of writing [5]. Notably, SARS-CoV-2 also appears to infect ciliated bronchial epithelial cells and type II pneumocytes predominantly via the ACE2 receptor and is structurally similar to the SARS-CoV-1 [6]. Akin to previous coronavirus outbreaks, SARS-CoV-2 likely originated from the bat, and the outbreak was initiated from animal-to-human transmission in markets in which wild animals were sold [4, 7].
Whilst the prevalence of diabetes in people infected with SARS-CoV-2 is relatively low compared to the population prevalence of diabetes in Chinese and Italian cohorts [8, 9], people with diabetes appear to develop more severe forms of the disease. Data from the UK suggest that a diagnosis of diabetes is a major risk factor for hospital admission in people with COVID-19, as 19% of 16,749 patients hospitalised with COVID-19 between February and April 2020 had underlying diabetes [10] (see Table 1). Indeed, one-third of all people dying from COVID-19 in the UK had an underlying diagnosis of diabetes. Regrettably, the odds of in-hospital death in people with type 1 and type 2 diabetes (T2D) was 2.86 and 1.81 compared to people without diabetes, after adjusting for confounding variables [11]. Moreover, at the time of writing, an underlying diagnosis of diabetes is the commonest underlying health condition amongst those who have died of COVID-19 in the UK [12].
People with diabetes infected with SARS-CoV-2 in China and Italy have also required greater use of (intensive care unit [ICU]) support and/or mechanical ventilation and have had a higher mortality rate than people without diabetes [13]. Indeed, studies from both the USA and China report that diabetes is an independent predictor of death in people with COVID-19 and that mortality is up to 50% higher in this patient population than in people without diabetes [14]. Moreover, a recently published analysis of people with diabetes hospitalised for COVID-19 in France found that 29.0% of people attained a primary outcome measure of mechanical ventilation and/or death within 7 days of hospitalisation with COVID-19. Interestingly, the authors of this study reported that high body mass index was independently associated with the primary outcome [15]. In fact, around 40% of people hospitalised with COVID-19 in the USA had underlying diabetes or hyperglycaemia, with a more than fourfold greater mortality than those who did not [16]. Therefore, it would appear that diabetes may act as a marker for poor metabolic health that in turn increases the risk of severe disease in those infected with SARS-CoV-2, as several other high-risk medical conditions are associated with diabetes, including hypertension, obesity, ischaemic heart disease and chronic renal disease.
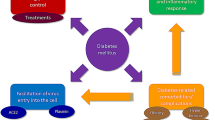
There is undoubtedly an increased mortality in people with diabetes and COVID-19, possibly due to the association of conditions with poor outcomes in COVID-19, such as hypertension, ischaemic heart disease and obesity [17], which are commonly observed in people with diabetes (see Fig. 1). Therefore, the mortality association may be confounded by this relationship. However, considerable biology links poor outcomes and higher mortality in people with diabetes and COVID-19. In this review we discuss the likely biological interactions between diabetes and COVID-19 and the potential impact of diabetes medications on COVID-19 severity and health outcomes.
The complex relationship between diabetes and coronavirus disease 2019 (COVID-19) mortality may be confounded by the association between diabetes and other risk factors for increased mortality in persons with COVID-19, including obesity, hypertension, increasing age, pro-inflammatory state, chronic respiratory disease, ethnicity and vitamin D deficiency. COPD Chronic obstructive pulmonary disease
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Diabetes and SARS-COV-2: Biological Interactions
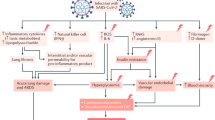
Diabetes is associated with poorer health outcomes as a result of its multi-system involvement and association with multiple cardiovascular, renal and other comorbidities, making it a leading cause of death worldwide [18]. Diabetes results in an inflammatory condition in which hyperglycaemia triggers the generation of pro-inflammatory cytokines resulting in oxidative stress and thereby tissue inflammation. Moreover, diabetes is associated with a relative immunodeficiency as a result of impaired macrophage and neutrophil function, reduced lymphocyte proliferation and complement activation dysfunction [19]. As a result of this pro-inflammatory and immunodeficient state, higher morbidity and mortality has previously been associated with several bacterial and viral infections in people with diabetes [18, 20]. Predictably, uncontrolled glycaemia was previously associated with worse outcomes in people with diabetes during both the SARS-CoV-1 [21] and MERS-CoV [22] disease outbreaks. However, there remains debate on whether hyperglycaemia acts to exacerbate the infection or is simply a consequence of the stress response that results from coronavirus infection.
Owing to the high prevalence of people with diabetes and COVID-19 requiring hospitalisation and/or ICU support, there must be more specific mechanisms which affect people with diabetes infected with SARS-CoV-2 [14]. Given our understanding of the mechanism of SARS-CoV-1 and MERS-CoV, we review the potential role of ACE2 and DPP-4 receptors in the pathogenesis of SARS-CoV-2 in people with diabetes.
ACE2 Receptors
The ACE2 receptor is widely expressed within the epithelial cells of the respiratory tract, tubular epithelial cells of the renal tract, mucosal cells of the gastrointestinal tract, arterial and venous endothelial cells, cardiac myocytes and the pancreatic β-cells [17, 23]. Within the respiratory system, ACE2 converts angiotensin II to angiotensin I and, therefore, inhibition of ACE2 leads to elevated concentrations of angiotensin II, thereby generating a pro-inflammatory response and stimulating aldosterone secretion. Collectively, these actions enhance local vascular permeability and renal fluid retention, thus engendering respiratory distress [24]. SARS-CoV-2 enters the host cell utilising the envelope spike glycoprotein on its surface to bind the ACE2 enzyme which in turn modulates the enzyme’s activity, potentiating cell damage and respiratory failure [25]. Consequently, hypokalaemia has been noted as a feature of people who are critically ill from COVID-19 and is believed to be a result of renal potassium wasting from excess aldosterone secretion. Moreover, early normalisation of serum potassium is a predictor of an improved prognosis in people who are critically unwell with COVID-19 [26]. Interestingly, one recent study concluded that SARS-CoV-2 may cause acute kidney injury via the ACE2 receptor, as the virus was detected within human glomerular cells at autopsy [27].
In people with diabetes, hyperglycaemia in the earlier stages of COVID-19 may worsen disease outcomes as hyperglycaemia induces the glycosylation of the ACE2 receptor, promoting cellular linkage to the SARS-CoV-2 virus and therefore promoting infection of the host cell and causing a higher disease severity [28]. Moreover, early correction of hyperglycaemia can reverse this process and may improve disease outcomes, leading many authors to argue a case for tight glycaemic control as a priority in the care for people with diabetes who have COVID-19 [17, 29,30,31]. In addition to enhanced ACE2 receptor glycosylation, upregulation of ACE2 receptor expression may enhance the ability of SARS-CoV-2 to enter and infect the host cell. As a result, there are safety concerns surrounding the use of many medications which are commonly used in people with diabetes, including ACE inhibitors, angiotensin-receptor blockers, ibuprofen and thiazolidinediones, all of which appear to increase the expression of ACE2 [17]. Nevertheless, little evidence currently supports their discontinuation in routine practice, and current guidance supports continuation of these medications [17, 32]. However, further study into the possibly harmful effect of such frequently utilised medications in people with diabetes and cardiovascular disease is warranted [33].
Genetic analyses of the ACE2 gene provide further explanation for the poorer health outcomes seen in people with COVID-19 and diabetes. The ACE2 enzyme is expressed by the ACE2 gene on the X chromosome [34]. Interestingly, expression of ACE2 has been linked with the development of T2D, and the expression of the single nucleotide polymorphism G8790A on the ACE2 gene also increases the risk of developing T2D [35, 36]. Theoretically, an increased expression of ACE2 or specific ACE2 polymorphisms may result in greater disease severity by increased binding potential with the SARS-CoV-2 virus. One study observed that Asian men had greater expression of ACE2 than their White or African American counterparts [37], implying that this may explain some of the population differences observed in COVID-19 severity. Additionally, Cao and colleagues observed that East Asian populations had higher ACE2 receptor tissue expression, which again may explain differences in susceptibility or response to SARS-CoV-2 [38]. However, further investigation into this potential risk factor is warranted [33] and may lead to further therapeutic targets.
Further interest in the role of ACE2 in people with diabetes comes from studies identifying this receptor in pancreatic β-cells [39]. Recent observations of greater incidence rates of diabetic ketoacidosis have been reported in people with or without an underlying diagnosis of diabetes and are associated with a high mortality rate [40]. Diabetic ketoacidosis in this setting likely results from the destruction of pancreatic β-cells following SARS-CoV-2 entry into the cell via the ACE2 receptor. Indeed, people with insulin-treated diabetes and COVID-19 appear to have greater insulin resistance and require significantly higher insulin doses [14]. This may simply reflect the severity of infection inducing a considerable catecholamine response which generates greater insulin resistance or it is a direct mechanism in which SARS-CoV-2 further impairs β-cell function. The latter argument is supported by a previous study conducted during the SARS-CoV-1 outbreak which observed that approximately 50% of non-diabetic patients with SARS-CoV-1 infection developed diabetes as an inpatient, although only 5% of participants had diabetes at follow-up 3 years later. The authors reason that as SARS-CoV-1 binds to the ACE2 receptor in the pancreatic β-cells, the resulting damage to the islet cells impairs insulin release [39]. It is this relative hypoinsulinaemia that is thought to be the cause of the higher incidence of hyperglycaemia and diabetic ketoacidosis in these patients [40]. As a result, many argue that tighter blood glucose control is essential and will improve health outcomes in people with diabetes [17, 29,30,31].
DPP-4 Receptors
The interest in DPP-4 receptors started with the finding that the MERS-CoV virus targeted this receptor to enter and subsequently infect a host cell [14] and that antibodies directed against DPP-4 inhibit the infection of host cells by the MERS-CoV virus [41]. Of course, the DPP-4 enzyme plays a critical role in glucose metabolism by degrading incretin hormones, such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide, resulting in reduced insulin secretion [42]. However, DPP-4 (also known as cluster of differentiation-26 [CD26]) plays an additional immunomodulatory role by activating T cells and maintaining lymphocyte function [43]. Recent studies have observed that the DPP-4/CD26 molecule also interacts with the S1 domain of the SARS-CoV-2 viral spike glycoprotein [44], in addition to the initially reported interaction with the ACE2 receptor [6]. Moreover, there are structural similarities between SARS-CoV-2 and the MERS-CoV and SARS-CoV-1 viruses [4, 44]. However, results are mixed, and other studies do not support an interaction between SARS-CoV-2 and cells expressing DPP-4, but only to cells expressing ACE2 [45]. Nevertheless, there remains a potential role for DPP-4 inhibitors in the treatment of people with COVID-19, leading some to call for further studies to explore this potential therapeutic avenue [46, 47].
Diabetes Pharmacotherapy and SARS-COV-2: Therapeutic Interactions of Sodium–Glucose Cotransporter-2 Inhibitors
Drugs within this class of medication inhibit the sodium–glucose cotransporter-2 (SGLT-2) protein in the proximal convoluted tubule, thereby preventing the reuptake of both sodium and glucose to promote their urinary excretion [48]. Use of these medications is associated with significant improvements in glycaemic control, body weight and reduced progression of renal disease [49, 50]. Since their introduction in 2013, their prescribing usage has increased dramatically, and they are amongst the most commonly used drugs for treating T2D [51].
There is strong rationale for the use of SGLT-2 inhibitors as a potential treatment for people with COVID-19. Firstly, the use of these medications promotes diuresis and natriuresis, which improves heart failure outcomes [52, 53]. Additionally, their use appears to selectively reduce interstitial fluid volume without significantly affecting the intravascular fluid volume [54]. Collectively, these actions may improve COVID-19 health outcomes because, as discussed above, COVID-19 locally increases vascular permeability within the lung, thereby increasing interstitial fluid volume and consequently inducing respiratory distress [24]. Secondly, the use of SGLT-2 inhibitors is associated with a shift in myocardial and renal metabolism from carbohydrate and/or lipids to ketones which are more energy-efficient and may improve both myocardial and renal function [55]. Thirdly, SGLT-2 inhibitors are also believed to exert an anti-inflammatory effect by reducing serum leptin, tumour necrosis factor-alpha (TNF-α) and interleukin 6 (IL-6) levels but increasing serum adiponectin level [56]. Given the pro-inflammatory state associated with diabetes [19], reducing such inflammatory markers may result in improved morbidity and mortality associated with COVID-19. Indeed, the use of SGLT-2 inhibitors appears to be associated with favourable cardiovascular and general health outcomes, making them an attractive therapeutic option in people with diabetes and COVID-19.
As a result, studies to establish whether these drugs improve COVID-19 outcomes in people with or without diabetes would be important. The DARE-19 trial is a phase III randomised, double-blind and placebo-controlled trial which aims to establish whether dapagliflozin 10 mg reduces disease progression, complications and/or all-cause mortality in people with COVID-19 [57]. Interestingly, not all of the study participants will have a diagnosis of diabetes, but there is a requirement of a diagnosis of either hypertension, T2D, atherosclerotic cardiovascular disease, heart failure or chronic renal disease to participate [57]. We are unaware of other clinical trials investigating the use of these medications at the time of writing.
However, there are some major concerns surrounding the use of SGLT-2 inhibitors in people with diabetes and COVID-19. Such concerns are due to the increased risk of diabetic ketoacidosis associated with the use of SGLT-2 inhibitors as a result of increased urinary glucose excretion, with reversal of the insulin:glucagon ratio which then precipitates ketosis [58]. Given the increased risk of ketoacidosis in people with COVID-19 discussed above, there is therefore potential for harm associated with the use of SGLT-2 inhibitors, and the current recommendation is to withhold these drugs during illness, in keeping with sick day rules [14, 59]. Moreover, SGLT-2 inhibitors promote renal ACE2 activity, and thus there is potential that the use of these medications may promote SARS-CoV-2 infectivity [60]. However, there are no trials to date which demonstrate this, and further data are required. We await the outcome of the DARE-19 trial with anticipation to further investigate the benefits and potential risks associated with the use of dapagliflozin.
Incretin-Based Therapies
DPP-4 Inhibitors
As discussed above, there is considerable interest in the DPP-4 receptor as a therapeutic target in people with COVID-19 as a result of its potential interaction with this receptor to infect a host cell [44]. Whilst there is no currently available clinical data to support this, some studies are underway to examine the impact of DPP-4 inhibition on COVID-19 outcomes in people with diabetes. The aim of an observational, retrospective case–control study in Italy is to compare clinical and biochemical outcomes in people with diabetes treated with the DPP-4 inhibitor sitagliptin to people with diabetes not treated with sitagliptin [61]. The objective of a randomised, open-label trial is to compare measures of glycaemic control, inflammation and chest x-ray findings between insulin-treated patients with T2D with or without the DPP-4 inhibitor linagliptin [62]. At the time of writing, no further clinical trials on DPP-4 inhibitors were found.
GLP-1 Analogues
Glucagon-like peptide-1 is an endogenous peptide secreted by the distal small bowel in response to glucose ingestion to stimulate insulin release from the β-cells of the pancreas and is subsequently degraded by the DPP-4 enzyme. GLP-1 analogues act directly to stimulate pancreatic insulin secretion and are associated with improved glycaemic control, body weight and reduced progression of renal disease [49, 50].
GLP-1 analogues may interact with the COVID-19 disease process through two key processes; upregulation of ACE2 receptors and anti-inflammatory effects. In rats, use of the GLP-1 analogue liraglutide was associated with upregulation of ACE2 receptors in the cardiopulmonary system [63] and the liver [64]. Secondly, the use of GLP-1 analogues is known to improve metabolic health generally at least partly through reducing endovascular inflammation [65]. Indeed, one pre-clinical trial found that use of liraglutide in rats was associated with reduced inflammatory measures, including macrophage infiltration, and reduced TNF-α and IL-6 levels [66]. However, there is a paucity of evidence from human studies in this area [60], and we are not aware of any ongoing trials examining the potential impact of these medications of COVID-19 outcomes at the time of writing this review.
Conclusions
COVID-19 is a worldwide pandemic which has resulted in an extraordinary number of infections and deaths worldwide. People with diabetes are particularly vulnerable to develop more severe forms of the disease, possibly due to a clustering of risk factors, such as hypertension, ischaemic heart disease and chronic renal disease. Common pathways appear to exist that may explain the susceptibility of this patient population to COVID-19, and some medications commonly used for people with diabetes may interact with these pathways. However, little is known to date on whether these interactions lead to clinical harm or benefit or indeed whether they have any impact at all, although a recent analysis of people with diabetes hospitalised in France with COVID-19 demonstrated no association with the need for mechanical ventilation or death within 7 days with glucose-lowering therapies such as metformin and insulin [15]. Safe trials exploring such medications are welcomed, and we hope these will shed light on this rapidly evolving area in a time of great uncertainty whilst we continue to rapidly learn more about this novel disease.
References
Zhong NS, Zheng BJ, Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February 2003. Lancet. 2003;362(9393):1353–8.
Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–20.
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–92.
Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3.
John Hopkins University of Medicine. Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html. Accessed 26 May 2020.
Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–9.
Khan S, Siddique R, Shereen MA, et al. Emergence of a novel coronavirus, severe acute respiratory syndrome coronavirus 2: biology and therapeutic options. J Clin Microbiol. 2020;58(5):e00187–e220.
Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8.
Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020. https://doi.org/10.1007/s40618-020-01236-2.
Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.04.23.20076042v1. Accessed 16 May 2020.
Barron E. Bakhai C. Kar P, et al. Type 1 and Type 2 diabetes and COVID-19 related mortality in England: a whole population study. NHS England. 2020. https://www.england.nhs.uk/publication/type-1-and-type-2-diabetes-and-covid-19-related-mortality-in-england/. Accessed 26 May 2020.
NHS England. Statistics: COVID-19 Daily Deaths. 2020. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-daily-deaths/. Accessed 16 May 2020.
Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354.
Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–50.
Cariou B, Hadjadi S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetelogia. 2020. https://diabetologia-journal.org/wp-content/uploads/2020/05/20-0610-Cariou-in-press2.pdf.
The Lancet Diabetes & Endocrinology. COVID-19: underlying metabolic health in the spotlight. Lancet Diabetes Endocrinol. 2020;8(6):457. https://doi.org/10.1016/S2213-8587(20)30164-9.
Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142.
Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–58.
Knapp S. Diabetes and infection: is there a link? A mini-review. Gerontology. 2013;59(2):99–104.
Zoppini G, Fedeli U, Schievano E, et al. Mortality from infectious diseases in diabetes. Nutr Metab Cardiovasc Dis. 2018;28(5):444–50.
Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–8.
Banik GR, Alqahtani AS, Booy R, Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31(1):81–4.
Song Z, Xu Y, Bao L, et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59.
Bornstein SR, Dalan R, Hopkins D, Mingrone G, Boehm BO. Endocrine and metabolic link to coronavirus infection. Nat Rev Endocrinol. 2020;16(6):297–8.
Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8.
Chen D, Li X, Song Q, Hu C, Su F, Dai J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). medRxiv. 2020. https://medrxiv.org/lookup/doi/10.1101/2020.02.27.20028530. Accessed 16 May 2020.
Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020. https://doi.org/10.1056/NEJMc2011400.
Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract. 2020;163:108186.
Zhu L, She ZG, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–77.e3.
Zhou J, Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216.
Wang A, Zhao W, Xu Z, Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162:108118.
Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–81.
Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21.
Liu J, Ji H, Zheng W, et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1(1):6.
Chaoxin J, Daili S, Yanxin H, et al. The influence of angiotensin-converting enzyme 2 gene polymorphisms on type 2 diabetes mellitus and coronary heart disease. Eur Rev Med Pharmacol Sci. 2013;17(19):2654–9.
Ramachandran V, Ismail P, Stanslas J, et al. Association of insertion/deletion polymorphism of angiotensin-converting enzyme gene with essential hypertension and type 2 diabetes mellitus in Malaysian subjects. J Renin Angiotensin Aldosterone Syst. 2008;9(4):208–14.
Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. Bioryxiv. 2020. https://www.biorxiv.org/content/10.1101/2020.01.26.919985v1. Accessed 16 May 2020.
Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11.
Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–9.
Li J, Wang X, Chen J, et al. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020. https://doi.org/10.1111/dom.14057.
Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–4.
Deacon CF. A review of dipeptidyl peptidase-4 inhibitors. Hot topics from randomized controlled trials. Diabetes Obes Metab. 2018;20(S1):34–46.
Klemann C, Wagner L, Stephan M, von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol. 2016;185(1):1–21.
Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601–4.
Tai W, He L, Zhang X, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020;17(6):613–20.
Iacobellis G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162:108125.
Strollo R, Pozzilli P. DPP4 inhibition: preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. 2020. https://doi.org/10.1002/dmrr.3330.
Musso G, Gambino R, Cassader M, Pagano G. A novel approach to control hyperglycemia in type 2 diabetes: sodium glucose co-transport (SGLT) inhibitors: systematic review and meta-analysis of randomized trials. Ann Med. 2012;44(4):375–93.
Williams DM, Nawaz A, Evans M. Renal outcomes in type 2 diabetes: a review of cardiovascular and renal outcome trials. Diabetes Ther. 2020;11:369–86.
Williams DM, Nawaz A, Evans M. Drug therapy in obesity: a review of current and emerging treatments. Diabetes Ther. 2020;11(6):1199–1216.
Dennis JM, Henley WE, McGovern AP, et al. Time trends in prescribing of type 2 diabetes drugs, glycaemic response and risk factors: a retrospective analysis of primary care data, 2010–2017. Diabetes Obes Metab. 2019;21(7):1576–84.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–288.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin–angiotensin system. Int J Mol Sci. 2019;20(3):629.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–22.
Bonnet F, Scheen AJ. Effects of SGLT2 inhibitors on systemic and tissue low-grade inflammation: the potential contribution to diabetes complications and cardiovascular disease. Diabetes Metab. 2018;44(6):457–64.
ClinicalTrials.gov. Dapagliflozin in respiratory failure in patients with COVID-19 (DARE-19). 2020. https://clinicaltrials.gov/ct2/show/NCT04350593. Accessed 16 May 2020.
Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38(9):1638–42.
Diabetes UK. Updates: Coronavirus and diabetes. 2020. https://www.diabetes.org.uk/about_us/news/coronavirus. Accessed 16 May 2020.
Pal R, Bhadada SK. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res Clin Pract. 2020;163:108146.
ClinicalTrials.gov. Sitagliptin treatment in diabetic COVID-19 positive patients (SIDIACO-RETRO'). 2020. https://clinicaltrials.gov/ct2/show/NCT04382794. Accessed 16 May 2020.
ClinicalTrials.gov. Effects of DPP4 Inhibition on COVID-19. 2020. https://clinicaltrials.gov/ct2/show/NCT04341935. Accessed 16 May 2020.
Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–69.
Yang M, Ma X, Xuan X, et al. Liraglutide attenuates non-alcoholic fatty liver disease in mice by regulating the local renin-angiotensin system. Front Pharmacol. 2020;11:432.
Rizzo M, Nikolic D, Banach M, et al. Incretin-based therapies, glucometabolic health and endovascular inflammation. Curr Pharm Des. 2014;20(31):4953–60.
He J, Yuan G, Cheng F, Zhang J, Guo X. Mast cell and M1 macrophage infiltration and local pro-inflammatory factors were attenuated with incretin-based therapies in obesity-related glomerulopathy. Metab Syndr Relat Disord. 2017;15(7):344–53.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
David M Williams and Asif Nawaz have nothing to disclose. Marc Evans received financial support for consultancy from Novartis, Merck Sharp & Dohme Corp. and Novo Nordisk and has served on the speaker’s bureau for Novartis, Lilly, Boehringer lngelheim, Merck Sharp & Dohme Corp., Novo Nordisk, Janssen and Takeda. Marc Evans is also the Editor-in-Chief of Diabetes Therapy.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to: https://doi.org/10.6084/m9.figshare.12445106.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Williams, D.M., Nawaz, A. & Evans, M. Diabetes and Novel Coronavirus Infection: Implications for Treatment. Diabetes Ther 11, 1915–1924 (2020). https://doi.org/10.1007/s13300-020-00858-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00858-2