Abstract
Purpose
The aim of this study is to demonstrate that the total number of days in hospital required for healing of a de novo diabetes-related foot ulcer (DFU) is lower in patients followed up using a telemedicine platform (Télépied Follow-Up group [Group 2]) than in patients followed up using standard care (Standard Follow-Up control group [Group 1]). Patients are assigned to either Group 1 or Group 2 depending on whether their first inclusion visit is during an even or odd week. Patients included in Group 1 are to be followed at spaced intervals during day hospital visits by the investigator assisted by a specialized referral nurse as part of the regular follow-up procedure (dressing changes + ulcer monitoring). Between visits, an independent nurse (IN) provides local care on a daily basis. Patients included in Group 2 have their DFU treated by a referral nurse trained at the diabetic foot unit of the investigating centre, and they are also followed up by an IN under the supervision of a referral nurse. In Group 2, monitoring of lesions is performed weekly by the referral nurse using photos of the DFU with planimetry taken by the IN and sent to the referral nurse via telemedicine software. The referral nurse can, in turn, provide guidance to the IN on the care to be provided and/or decide that a further hospital visit is needed. Both treatment groups are to be followed for 12 months or until complete healing of the ulcer.
Results
Recruitment for the study began in March 2017 and ended in May 2019, with the final study visit scheduled for May 2020.
Conclusion
The aim of the Télépied study is to assess the impact of ambulatory foot ulcer management in diabetics over a 1-year period by a non-specialized IN working under the supervision of a referral nurse via telemedicine follow-up versus standard follow-up by an IN alone. The primary endpoint is the total duration of hospitalization required until full healing of the ulcer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Diabetic foot ulcer (DFU) is a costly complication of diabetes, with the extent of costs related to the frequency and duration of hospitalizations, amputations, ambulatory follow-up, transportation costs and disruptions to daily working activities. |
Telemedicine has been extensively studied for the management of diabetic subjects in general and has also proved to be effective in reducing and improving glycaemic balance in diabetic patients; however, very few telemedicine studies on the management of DFU have been conducted to date. |
The main objective of this study is to assess whether telemedicine can significantly reduce the number of days of hospitalization associated with foot ulcers in diabetic subjects. |
What was learned from the study? |
The design of the telepied study is described; recruitment for the study ended in May 2019 with a total of 180 patients. |
Context
Diabetic foot ulcer (DFU) is a major public health issue worldwide in patients with diabetes [1]. It is associated with poorer survival and functional outcomes and a high recurrence rate [2]. DFU is one of the leading causes of lower limb amputation [3], with some 50% of non-traumatic amputations being performed in diabetic patients [4, 5]. It is also characterized by slow and difficult healing and associated with a high risk of amputation and infectious complications [6], as evidenced by such patients having a mortality rate at 5 years equivalent to that of colon cancer patients [7]. DFU is a complication associated with high treatment costs [8], with the extent of costs related to the frequency and duration of hospitalizations, need for amputation, ambulatory follow-up, transportation costs and disruption to daily working activities (with the latter costs also corresponding to one of the indirect costs of DFU management). A survey conducted by the Institute for Health Watch reported that, in France, the number of hospitalizations associated with DFU increased by fivefold in the 10 years between 1997 and 2007.
The use of telemedicine in general for the management of diabetic subjects has been extensively studied [9]. More specifically, telemedicine has been proven to be effective in reducing and improving glycaemic balance in diabetic patients [10]. However, very few telemedicine studies have been conducted to date on the management of DFU [11]. A systematic review shows that the application of telehealth and telemedicine approaches for the management of DFU is still in its infancy [12], but this method seems to be more appreciated by and more satisfying to patients [13].
The main objective of the study described here is to assess whether telemedicine and management by a referral nurse can significantly reduce the number of days of hospitalization associated with foot ulcers in diabetic subjects. We believe an assessment of the combination of these two treatment strategies for patients with DFU is more appropriate than an assessment of the impact of telemedicine alone versus standard care.
Description and Rationale of the Study Method
This is a controlled single-centre study in open, parallel groups of diabetic patients with de novo foot ulcer or recurrence of a previously healed ulcer.
The study protocol was approved by the French National Agency for Medicines and Health Products Safety (ANSM) and by the ethics committee of Pitie Salpetrière University Hospital (Institutional Review Board; Agreement of US Department of Health and Human Services No. RCB:2016-A01136-45). Comprehensive information on the study was provided to each patient on a form printed specifically for this purpose, who must then provide written informed consent before enrolment.
Study Objectives
Main and secondary objectives
The main study objective is to show that the total number of days of hospitalization to healing of a de novo foot ulcer in a diabetic patient is lower in the experimental Télépied Follow-Up group (Télépied group) than in the Standard Follow-up control group.
The secondary objectives are: (1) to show that the healing time for DFU is not higher in the Télépied group than in the Standard Follow-Up group and (2) to compare the total cost of direct care for the Télépied group versus the Standard Follow-up control group.
Primary Evaluation Criterion
The primary evaluation criterion was comparison of the total number of days of hospitalization over 1 year or until complete healing of the DFU in the Télépied group versus the Standard Follow-Up group. Patients are hospitalized if they present aggravated ulcers requiring hospitalization and characterized by one or more of the following factors: (1) increased lesion area or depth; (2) appearance of signs of local infection; (3) signs of generalized infection.
Participants
Inclusion Criteria
The inclusion criteria are: (1) patients with type 1 or type 2 diabetes aged > 18 years who have been hospitalized or who have been seen by attending physicians in the diabetology ward for either a de novo foot ulcer or recurrence of a previously healed ulcer; (2) patients who have agreed to take part in the study and have provided signed informed consent; (3) patients whose healthcare is covered by social security.
Non-inclusion Criteria
Pregnant women, patients deprived of liberty by judicial or administrative decision, persons subject to legal protection measures or patients taking part in another clinical trial were excluded from participating in the study.
Description of the Study Tools
Photography
In both groups, the referral hospital nurse takes a picture of the ulcer at each hospital visit after cleansing the ulcer and providing local care. In the Télépied study group, the independent nurse also takes a photograph of the foot ulcer being investigated in the study during home visits conducted every 1 or 2 weeks. All photographs of ulcers will contain a ruled edge subdivided into millimetres that enables calibration of the photograph using the measuring software.
Measurement of Ulcer Area
The area of each plantar ulcer will be measured and calculated using Tracer.exe software developed by the University of Glamorgan (Pontypridd, Wales, UK) [14].
Adjudication Committee
The adjudication committee will have two main tasks: (1) to assess the course of ulcer healing and (2) to evaluate the therapeutic decisions made by the investigating doctor in order to eliminate any bias that the investigator might introduce with regard to the primary evaluation criterion.
Each ulcer photograph for a given patient will be analysed independently by the members of the adjudication committee. A consensus decision will then be made between the three members of the adjudication committee. Each therapeutic decision will be independently evaluated by the adjudication committee members who will determine whether or not the decision is warranted in the light of progress in treatment of the ulcer or in the patient's state of health.
Complete healing of the DFU investigated in the study is defined as complete epithelialization of the ulcer once the possibility of hyperkeratosis has been ruled out.
Study Conduct
Selection Visit
During their visits with the investigator at regular consultations or during hospitalization in the diabetes department, patients meeting the inclusion criteria are invited to take part in the study. If necessary, a cooling-off period may be considered, and patients may return to the centre later for the inclusion visit. For patients agreeing to participate in the selection visit, inclusion can be completed on the same day.
Inclusion Visit (V0)
Patients receive information from the investigating doctor on the study aims and conduct. They are provided with the patient information sheet and the informed consent form. They are asked to read the information sheet and then to date and sign the consent form prior to participating in any study-related procedures.
Patients included are assigned to a follow-up group based on the following defined schedule: patients presenting for their first visit during an odd week will be assigned to the Standard Follow-Up control group (Group 1); patients presenting for their first visit during an even week will be assigned to the Télépied Follow-Up study group (Group 2). Allocation to groups is to occur in an alternating fashion throughout the study inclusion period, i.e. a period of 6 months.
Key Data Collected
The key data collected are the inclusion criteria, non-inclusion criteria, demographic data, clinical data (glycated haemoglobin, current treatment, systolic/diastolic blood pressure, disease history, diabetes complications, description of the ulcer being followed up in the study, photograph of the ulcer with planimetric measurement of its area and estimated depth and presence of infection or ischaemia.
Standard Follow-Up Control (Group 1)
At the end of their V0 inclusion visit, during hospitalization or during a consultation visit, patients will receive follow-up visits to monitor their ulcers at a rate determined by the investigating doctor. At each visit, the investigating doctor performs a standard follow-up procedure.
Between hospital visits, patients may be assigned to one of the three subgroups defined by type of care:
-
Subgroup 1: Patients return home. Daily local ulcer care is given by an independent nurse.
-
Subgroup 2: Patients are followed up during hospitalization.
-
Subgroup 3: Patients are followed up at a follow-up and rehabilitation unit.
The follow-up period will be 12 months even if the ulcer is healed before this period
Télépied Follow-Up Study (Group 2)
Starting from their inclusion in the study, patients in the Télépied group are cared for by a referral nurse specially trained in diabetic foot ulcers.
At the end of their V0 inclusion visit, during hospitalization or during a consultation visit, patients will be assigned to one of the following three subgroups:
-
Subgroup 1: Patients return home. Daily local foot ulcer care is given by an independent nurse.
-
Subgroup 2: Patients are followed up during hospitalization.
-
Subgroup 3: Patients are followed up at a follow-up and rehabilitation unit.
After their inclusion visit, patients hospitalized in the diabetes ward are followed by the referral nurse to arrange their discharge. Patients included during a consultation return home after the inclusion visit. A “planning” visit is scheduled by the referral nurse on discharge day + 1. This visit allows a personalized care plan to be put in place as well as consultation on methods for the transmission of information between the independent nurse responsible for local ulcer care at the patient's home and the referral nurse performing a follow-up visit every 7 or 15 days at the patient's home.
At each of these visits, the independent nurse provides local care for the patient's foot ulcer.
Once a week at the time of local care and dressing changes the independent nurse and/or patient must provide the referral nurse with a photograph of the foot ulcer being studied.
Role of the Referral Nurse
-
participating in informing/training patients on the management of their ulcer (discharge, daily care, etc.),
-
monitoring patients for the duration of their hospitalization and organizing any necessary examinations as well as patient discharge from the hospital,
-
arranging patients’ discharge from hospital (whether returning home or other accomodation),
-
assisting patients on their return home by making the arrangements for follow-up with an independent nurse,
-
acting remotely as a reference for independent nurses who require advice or guidance in making care decisions,
-
providing support to expert doctors at the hospital,
-
organizing appointments and planning patient follow-up (additional examinations, re-hospitalization, consultations, etc.) together with the administrative staff in charge of coordinating consultations at the hospital,
-
centralizing reporting by the various independent nurses and managing the transmission of information to medical experts,
-
completing case report forms for the study.
Role of the Investigating Doctor
-
carrying out patient recruitment and supporting the referral nurse regarding medical follow-up,
-
providing support for the referral nurse,
-
validating the reports prepared by the reference nurse.
Data Analysis
Randomization: Assignment to a Follow-Up Group
Patients are not to be randomly assigned to one of the two groups but rather to be allocated according to a pre-decided schedule. Patients are automatically assigned to one or other of the groups studied according to the week of their first visit, with patients attending their first visit during an odd week assigned to the Standard Follow-up control group (Group 1), and those attending their first visit during an even week assigned to the Télépied Follow-up study group (Group 2). This methodology allows the referral nurse responsible for monitoring patients in the Télépied group to be present only when Group 2 patients are included, thereby avoiding potential bias or influence with regard to Group 1 patients, who are to be included in the study without the presence of the referral nurse. The allocation will also be made by block of predefined size. Randomization will be stratified according to the ankle-brachial index (ABI) measured during the inclusion visit, into three groups defined according to ABI > 0.9, 0.7 < ABI < 0.9 and ABI < 0.7. This stratification will make it possible to evenly distribute the patients suffering from severe arterial disease into the two treatment groups.
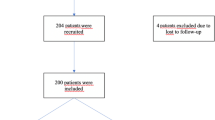
There are to be 180 evaluable patients.
The primary endpoint of the study as defined in the preceding text is the length of hospitalization during the post-hospital year for diabetic patients with DFU. Post-hospital follow-up of 185 patients with DFU resulted in a mean ± standard deviation of 27.25 ± 28.77 days of hospitalization per patient during the first year of follow-up. This mean and its standard deviation were taken as a reference for the control group in determining statistical power.
With the number of patients included in the analysis set at N = 90 per group and an alpha risk of 0.05 (two-sided), the following list shows the differences that can be detected between the two treatment groups with a statistical power of > 80% and different observed standard deviation:
-
a difference of 9 days for an overall standard deviation of 21,
-
a difference of 10 days for an overall standard deviation of 23,
-
a difference of 11 days for an overall standard deviation of 26,
-
a difference of 12 days for an overall standard deviation of 28.
Data Analysis Strategy
A detailed analytical plan will be prepared before the database is frozen and validated by the study sponsor and the principal investigator.
Evaluable Populations
-
Evaluable population: all randomized patients with at least one item of follow-up data.
-
Population evaluable for efficacy criteria (per-protocol population): all randomized patients presenting no major deviations.
Protocol deviations will be assessed during a blind review in order to define their severity and, based on their severity, a decision will be made on whether or not a given observation should be retained in the data analysis set.
General Remarks on Descriptive Analyses
All evaluation criteria will undergo a group-by-group analysis in terms of the following statistics:
-
For quantitative variables: number of missing values, number of non-missing values, mean, standard deviation, median, first quartile, third quartile, minimum and maximum.
-
For qualitative variables: number of missing values, number of non-missing values, frequencies, percentages for each modality of the variable (excluding missing data for the denominator).
Analysis of the Main Objective
The main objective of the study is to assess the superiority of the management of DFU via telemedicine versus standard follow-up.
Comparison of the mean number of days of hospitalization (associated with DFU) in each treatment group is to be tested using a variance analysis model that includes the stratification parameter. The null hypothesis tested will be equal distribution between the two groups of the number of days of hospitalization (associated with DFU) during the year following hospitalization.
The number of days are to be assessed for each group with a two-sided 95% confidence interval.
Analysis of Secondary Criteria
The means of the secondary quantitative criteria are to be compared using an analysis of variance model (including the stratification parameter) between the Télépied group and the Standard Follow-Up Control group. Secondary qualitative criteria will be compared using a logistic regression model (including the stratification parameter) between the Télépied group and the Standard Follow-Up Control group.
The two-sided 95% confidence interval will also be determined for each of the following secondary criteria:
-
mean duration of hospitalization (for one DFU) for each patient,
-
frequency of ulcerations for each patient,
-
average duration of progression of ulceration for each patient,
-
rate of healing for the foot ulcer analysed in the study,
-
number of patients undergoing amputation during the 1-year follow-up period,
-
change in patient satisfaction score (Diabetes Treatment Satisfaction Questionnaire [DTSQ]).
The total real per-patient cost of direct care associated with the presence of a DFU is the sum of the following costs (hospitalization costs + transportation costs + nursing costs + independent nursing costs + cost of materials + costs of consultations with doctors in private practice).
Type-1 Alpha Risk
The risk level for type-1 error in the analysis is set at 0.05 for a two-sided situation.
Study Organization and Progress
A total of 180 patients were included in the study, with the first inclusion taking place on 25 March 2017 and the last on 06 May 2019. At the time of submission of this article, 120 patients have completed the study. The final visit is scheduled for 07 May 2020. The main results will be published in 2020. Monitoring is to be carried out by the Centre for Studies and Research for Intensification of Diabetes Treatment (Evry, France).
Discussion
In this report we describe the rationale and design of the Télépied single-centre trial in diabetic subjects. The aim of this study is to compare the total duration of hospitalization over a 1-year follow-up required to achieve complete healing of a DFU using telemedicine management and a referral nurse versus the standard care pathway.
DFU continues to be inadequately studied and poorly funded, despite its prevalence and severity and the high social and economic burdens associated to it [15]. The healthcare costs for patients with DFU are enormous and account for 25–30% of the total costs associated with all care provided to diabetic patients [16, 17]. In France, the hospitalization rate for diabetic foot continues to rise [18], despite the presence of centres throughout the country specializing in its management. In real life, patients with DFU have little personalized follow-up, either at the referral centre or at their home.
Conclusions
The aim of the Télépied study is to demonstrate that personalized follow-up and telemedicine can reduce the need for hospitalization of a diabetic patient with DFU and, if required, shorten the duration of hospitalization by providing personalized advice targeted towards patients’ individual profiles and the precise characteristics of their lesions.
References
Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–24.
Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233(6):485–91.
Muller IS, de Grauw WJ, van Gerwen WH, Bartelink ML, van Den Hoogen HJ, Rutten GE. Foot ulceration and lower limb amputation in type 2 diabetic patients in dutch primary health care. Diabetes Care. 2002;25(3):570–4.
Fosse S, Hartemann-Heurtier A, Jacqueminet S, Van Ha G, Grimaldi A, Fagot-Campagna A. Incidence and characteristics of lower limb amputations in people with diabetes. Diabet Med J Br Diabet Assoc. 2009;26(4):391–6.
Rasmussen BS, Yderstraede KB, Carstensen B, Skov O, Beck-Nielsen H. Substantial reduction in the number of amputations among patients with diabetes: a cohort study over 16 years. Diabetologia. 2016;59(1):121–9.
Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999;22(5):692–5.
Armstrong DG, Wrobel J, Robbins JM. Guest Editorial: are diabetes-related wounds and amputations worse than cancer? Int Wound J. 2007;4(4):286–7.
Halimi S, Benhamou PY, Charras H. Cost of the diabetic foot. Diabetes Metab. 1993;19[5 Suppl]:518–22.
Franc S, Borot S, Ronsin O, et al. Telemedicine and type 1 diabetes: is technology per se sufficient to improve glycaemic control? Diabetes Metab. 2014;40(1):61–6. https://doi.org/10.1016/j.diabet.2013.09.001.
Charpentier G, Benhamou PY, Dardari D, et al. The DIABEO software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care. 2011;34(3):533–9. https://doi.org/10.2337/dc10-1259.
Froekjaer J, Rasmussen BS, Bjerregaard MR, et al. A randomized controlled trial comparing telemedical and standard outpatient monitoring of diabetic foot ulcers. Diabetes Care. 2015;38:1723–9.
Hazenberg CE, Stegge WB, Van Baal SG, Moll FL, Bus SA, de Stegge AWB. Telehealth and telemedicine applications for the diabetic foot: a systematic review. Diabetes Metab Res Rev. 2020;36(3):1–11.
Tchero H, Noubou L, Becsangele B, Mukisi-Mukaza M, Retali GR, Rusch E. Telemedicine in diabetic foot care: a systematic literature review of interventions and meta-analysis of controlled trials. Int J Low Extrem Wounds. 2017;16(4):274–83.
Plassmann P. Measuring wounds. J Wound Care. 1995;4(6):269–72.
Armstrong DG, Kanda VA, Lavery LA, Marston W, Mills JL Sr, Boulton AJ. Mind the gap: disparity between research funding and costs of care for diabetic foot ulcers. Diabetes Care. 2013;36(7):1815–7.
Kantor J, Margolis DJ. Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis. Dermatol Surg. 2001;27(4):347–51.
Driver VR, Fabbi M, Lavery LA, Gibbons G. The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc. 2010;100(5):335–41.
Sandrine F-E, Laurence M-B, Agnès H-H. Hospitalization for podiatric complications in people pharmacologically treated for diabetes in France, in 2013. BEH. 2015;34–35:638–44.
Acknowledgements
Funding
No funding was received for the journal’s rapid service fee. The Télépied trial was funded by the French Diabetes Society, the Centre for Research and Studies for Intensification of Diabetes Treatment and Fondation URGO. The study received also funding from GENOPOLE.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
DD, AP, CG and SF participated in the design of the study; MB, EB, LD, EX, CR and LO performed the statistical analysis; DD, SF and GC conceived the study, participated in its design and coordination and helped to draft the manuscript.
Disclosures
Dured Dardari, Sylvia Franc, Guillaume Charpentier, Elise Bobony, Laetitia Demangeon, Marie Bouly, Ilham Xhaard, Laurent Orlando, Maria Alhajj, Kadijatou Ly Sall, Caroline Randazzo and Alfred Penfornis have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the French National Agency for Medicines and Health Products Safety (ANSM) and by the ethics committee of Pitie Salpetrière University Hospital (Institutional Review Board; Agreement of US Department of Health and Human Services No. RCB:2016-A01136-45). Comprehensive information on the study was provided to each patient on a form printed specifically for this purpose, who must then provide written informed consent before enrolment.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Trials.gov identifier: NCT02986256.
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12126657.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dardari, D., Franc, S., Charpentier, G. et al. Télépied Study: A Single-Centre Trial in Diabetic Subjects Comparing Total Duration of Hospitalization Over a 1-Year Period Required for Complete Healing of a Foot Ulcer Using Telemedicine Management and a Referral Nurse Versus the Standard Care Pathway. Diabetes Ther 11, 1419–1427 (2020). https://doi.org/10.1007/s13300-020-00821-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00821-1