Abstract
Introduction
The EDITION development program confirmed that insulin glargine 300 U/mL (Gla-300) provides comparable glycemic control to insulin glargine 100 U/mL (Gla-100) but with lower hypoglycemia risk. Our study aimed to evaluate the effectiveness of Gla-300 in everyday practice.
Methods
This one-arm, non-interventional study included patients with type 2 diabetes who were switched to Gla-300-based basal-bolus therapy (BBT) and followed for 6 months. Indications for switching included inadequate glycemic control and/or hypoglycemic events with the previous regimen.
Results
Overall 229 patients were included, with mean age of 60.9 years. All glycemic variables improved between baseline and 6 months significantly (mean ± standard deviation [SD] hemoglobin A1c [HbA1c] from 8.9 ± 1.5% to 7.5 ± 1.1%, fasting blood glucose from 9.5 ± 3.1 mmol/L to 7.0 ± 2.1 mmol/L, postprandial blood glucose from 12.0 ± 3.8 mmol/L to 8.9 ± 2.5 mmol/L). Gla-300 doses were increased and mealtime insulin doses were unchanged. Rates of both non-severe and severe hypoglycemic events decreased significantly compared to pre-study and 6-month follow-up periods. Patients switched because of elevated HbA1c had higher baseline HbA1c and greater decrease in HbA1c paralleled with increase in insulin doses compared to those switched because of hypoglycemia.
Conclusions
In day-to-day practice, switching from human insulin to Gla-300-based BBT resulted in significant improvement in glycemic control and decrease in hypoglycemia risk.
Similar content being viewed by others
Insulin glargine 300 U/mL was shown to improve glycemic control equally as well as insulin glargine 100 U/mL but was associated with lower risk of hypoglycemia. |
This non-interventional trial was initiated to investigate the effectiveness of insulin glargine 300 U/mL in subgroups of patients with type 2 diabetes specified in the Hungarian reimbursement rules. |
When switching from human insulin treatment, insulin glargine 300 U/mL based analogue basal-bolus regimen resulted in significant improvement in glycemic control and could be applied safely with decreased risk of hypoglycaemia. |
Insulin glargine 300 U/mL performed well in subgroup of patients switched due to inadequate glycemic control and in subgroup switched due repeated or serious hypoglycemic events. |
Introduction
The joint task force of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) updated its consensus on the management of type 2 diabetes (T2DM) in 2018 [1] reflecting both the epidemiologic and healthcare impact of the condition and the increasingly complex treatment algorithm that physicians need to apply in a personalized way at individual patient level. Echoing the consensus statements in 2012 and 2015 [2, 3], lifestyle modification composes the foundation of treatment followed by metformin monotherapy; then, in case of inadequate glycemic control, metformin can be supplemented with several other drugs, including basal insulin.
Long-acting basal insulin (BI) analogues have successfully been used for almost two decades. Insulin glargine 100 U/mL (Gla-100), the first member of this new drug class, was approved in 2000 [4]. Its clinical program demonstrated its prolonged effect compared to human BI, its relatively peak-less concentration–time profile, and consequently lower risk of hypoglycemia [4] associated with its use. As a result of the residual limitation of their duration of action to reliably cover 24 h, first-generation BI analogues have been further improved by manufacturers which resulted in the development of second-generation BI analogues, insulin glargine 300 U/mL (Gla-300) and insulin degludec.
In the EDITION clinical trial program (EDITION 1, 2, and 3 trials [5,6,7]), which included patients with T2DM, Gla-300 was compared to Gla-100 as part of different treatment regimens. In all these studies, the glycemic control (indicated by the levels of hemoglobin A1c [HbA1c]) improved equally in both study arms. From a safety perspective, the EDITION studies provided evidence that Gla-300 compared to Gla-100 was associated with lower risk of hypoglycemia, probably due to its improved pharmacokinetic and pharmacodynamics (PK/PD) properties [8, 9]. The 6-month patient-level meta-analysis of EDITION 1, 2, and 3 trials [10] confirmed the results of the individual trials, while the analysis of the 12-month data revealed a modest but statistically significant difference in HbA1c as well in favor of Gla-300 [11]. The lower risk of hypoglycemic events with Gla-300 was detected not only during highly controlled randomized clinical trials but also the analysis of data of patients with T2DM from the Optum Humedica database showed [12] that Gla-300 was associated with lower event rate of severe hypoglycemia when compared to first-generation BI analogues (Gla-100 and insulin detemir). This analysis also confirmed that the lower rate of severe hypoglycemia was not associated with worse glycemic control.
Insulin glargine 300 U/mL (Gla-300) was launched in Hungary in 2016 and has been mainly used as part of basal-bolus therapy (BBT) as a result of restricted national reimbursement conditions. For patients with T2DM, it can be prescribed with 50% patient co-payment in basal-only therapy or with 100% reimbursement as part of BBT. However, even in BBT, analogue insulins can be initiated as second-line options when (1) targeted glycemic control (i.e., HbA1c < 8%) could not be achieved after at least 3 months on human BBT; or (2) the patient had at least three documented hypoglycemic events per month during a 3-month human BBT period; or (3) the patient had at least one severe hypoglycemic event (an event that required third-party assistance) on human BBT. In addition to treatment initiation rules, a stopping rule for analogue insulin treatment has also been introduced. Gla-300 and other BI analogues could only be prescribed beyond 1-year treatment period if at least two HbA1c levels of below 8.0% have been measured during analogue BBT over the course of 12 months.
As there was no real-life experience with Gla-300 in Hungary, a non-interventional trial was initiated in 2016 to investigate its effectiveness (impact on glycemic control and hypoglycemia risk) in everyday practice in T2DM. The included patients met the Hungarian reimbursement rules, i.e., were switched from human BBT to Gla-300-based analogue BBT (on the basis of payer criteria defined above). The patients were administered insulin glulisine as mealtime insulin.
Methods
This prospective, one-arm, non-interventional study included patients with T2DM, aged 18–80 years, who were switched to insulin glargine 300 U/mL (Gla-300) plus insulin glulisine treatment at the time of inclusion. The patients had to meet at least one of the reimbursement criteria detailed in the “Introduction” section.
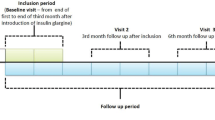
During the 6-month study period, the patients were followed according to local practice. Clinical parameters and laboratory results routinely collected in day-to-day care were recorded, with study-related visits at baseline (i.e., at enrollment—visit 1), at 3 months (visit 2), and at 6 months (visit 3), according to everyday practice. At baseline, recorded data included demographics, treatment and medical history, hypoglycemic events reported during the last 3 months before the switch to analogue treatment, current antidiabetic (insulin and oral antidiabetes agent [OAD]) treatment; reason (indication) for switching to analogue basal-bolus regimen; and glycemic parameters including target ranges for HbA1c, fasting plasma glucose (FPG), and postprandial blood glucose (PPG). At follow-up visits, current glycemic variables, body weight, current OAD treatments, insulin doses, and self-reported hypoglycemic and other safety events were recorded; fasting blood glucose and PPG levels were self-monitored by patients. The safety analysis included the patients who had at least one follow-up physician contact. The efficacy analysis included the patients who had available HbA1c values both at baseline and at 6 months. Additional subgroup analyses were conducted comparing patient subgroups according to their switching indication, i.e., inadequate glycemic control or hypoglycemic events on previous human insulin regimen. Those patients who were switched because of more than one indication were excluded from these subgroup analyses.
Normal distribution of continuous variables was investigated with a quantile–quantile (QQ) plot. Paired and two-sample t tests were applied for group comparisons and for time-dependent changes, respectively. All analyses were run in IBM SPSS Statistics 19.0.
The study (named “Toujeo-6M”) conformed to the Helsinki Declaration of 1964, as revised in 2013, and was approved by Hungarian National Institute of Pharmacy and Nutrition (registration code OGYI/44755-7/2015) and received national ethical approval as well. All patients gave written informed consent before any data collection.
Results
Baseline Characteristics
Overall 229 patients were included, of whom 217 patients (94.8%) had at least one follow-up visit (included in the safety analyses) and 189 (82.5%) had enough data to be included in the efficacy analyses. In the subgroup analyses (that excluded patients who were switched because of more than one indication), overall 190 patients could be included in the safety analyses (152 were switched because of inadequate control and 38 because of hypoglycemic events) and 160 in the efficacy analyses (of whom 128 were switched because of inadequate control and 32 because of hypoglycemic events).
Baseline characteristics for the efficacy population are summarized in Table 1. At baseline, patients’ mean age was 60.9 ± 10.4 years (± standard deviation [SD]), mean duration of T2DM was 13.4 ± 7.9 years, and mean treatment duration with insulin was 7.4 ± 6.2 years. The proportion of male patients was 47.1%. Mean body mass index (BMI) was 32.8 ± 5.8 kg/m2 at baseline.
Available glycemic parameters indicated inadequate glycemic control for the enrolled population. Baseline daily doses of both basal and prandial analogue insulins were higher compared to the applied human insulin doses before switch. The most frequently (58.7%) specified individual target HbA1c range was below 8.0% and at least 7.0%. Target HbA1c was not specified in one-quarter of the patients.
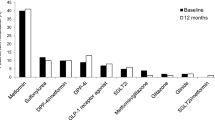
Among patients receiving OAD treatment at baseline (53.9% in total), 49.7% were receiving one OAD and 4.2% were receiving two OADs. The most frequently used OAD was metformin (53.4%), with empagliflozin (3.7%), glicazide (0.5%), and sitagliptin (0.5%) being used by a small proportion of patients.
Efficacy Outcomes, Target Range Achievement, and Change in Body Weight
The three glycemic variables significantly decreased by the end of the study (mean ± SD): HbA1c from 8.9 ± 1.5% to 7.5 ± 1.1%, fasting blood glucose from 9.5 ± 3.1 mmol/L to 7.0 ± 2.1 mmol/L, and postprandial blood glucose from 12.0 ± 3.8 mmol/L to 8.9 ± 2.5 mmol/L (Table 2). All of these improvements were statistically significant already by 3 months. Clinically relevant improvement between 3 and 6 months could be detected in HbA1c levels. Accordingly, the proportion of patients achieving different HbA1c target values (i.e., < 7% that is the most commonly specified in guidelines, and 8% that is the cutoff value in the Hungarian reimbursement rules) continuously increased, and close to 80% of patients achieved the 8.0% payer target by 6 months (Table 2). Body weight slightly decreased by 3 and 6 months as well.
Analogue Insulin Doses
While daily BI doses were increased from baseline to 6 months (from 34.5 ± 16.6 U to 41.0 ± 18.7 U), the daily prandial insulin doses remained virtually the same (Table 2).
Safety Outcomes
Data was collected (based on patients’ self-report) for confirmed non-severe hypoglycemic events (blood glucose ≤ 3.9 mmol/L and < 3.1 mmol/L), and for severe events when patients needed third-party assistance. Comparison of the 3-month period before inclusion (reference period) showed that the cumulative incidence (proportion of patients with at least one event) of both non-severe and severe events decreased from 44.2% to 27.6% and from 8.3% to 1.0%, respectively, although the length of study observation (6 months) was twice as long as the reference period before enrollment (3 months). The event rates (events/patient-year) were statistically significantly lower in both the first and the second 3-month periods, as well as during the whole study period compared to the reference period (Table 3).
Comparison of Patient Subgroups According to Switching Indications
On the basis of the indication for the switch to analogue insulin, the 190 patients could be categorized into subgroups switched because of a single reason of having either inadequate control (N = 152, uncontrolled subgroup) or hypoglycemic events (N = 38, hypo subgroup), of whom 128 and 32 patients had enough data (baseline and follow-up HbA1c) to be included in the subgroup efficacy analyses, respectively.
The baseline HbA1c level and the change in HbA1c during the study showed considerable differences according to switching indications. Patients who were switched because of inadequate glycemic control at baseline had significantly higher baseline HbA1c value and their improvement of glycemic control was larger compared to patients who were switched because of hypoglycemia. The hypo subgroup did not improve their glycemic control during the study; however, on the basis of their baseline HbA1c value, one would not have expected them to do so (Fig. 1). Baseline dose of Gla-300 was higher in the uncontrolled subgroup but the doses were increased significantly in both subgroups—although to a considerably larger extent in the uncontrolled subgroup (Fig. 2). Changes in body weight were not significant in any subgroup. Prandial insulin doses were higher in the uncontrolled subgroup at baseline and 6 months; however, changes were not significant in any subgroup (Table 4).
The cumulative incidence and event rate of all non-severe hypoglycemic events in the hypo subgroup well reflected the respective switching indication and were considerably higher compared to the uncontrolled subgroup (100% and 29.87 events/patient-year versus 17.8% and 2.74 events/patient-year, respectively). Remarkably the event rate (with ≤ 3.9 mmol/L hypoglycemia cutoff) in the hypo subgroup decreased to similar level compared to the event rate in the uncontrolled subgroup despite having a considerably higher rate at baseline before the switching (29.87 and 3.05 versus 2.74 and 2.0 events/patient-year, respectively). The frequency of non-severe hypoglycemic events with blood glucose level below 3.1 mmol/L was similar in both subgroups after analogue switch (Table 5).
Discussion
Insulin glargine 300 U/mL (Toujeo® [Gla-300]) was launched in Hungary in 2016; however, its 100% reimbursement rate is conditioned to complex payer requirements including inadequate glycemic control and/or occurrence of non-severe or severe hypoglycemic events during human BBT. Consequently, the patients switched to analogue BBT can be categorized according to the indication(s) for the switch. As “inadequate glycemic control” in the reimbursement rules is defined as HbA1c ≥ 8%, this value is an important cutoff point in daily clinical practice. This reimbursement defined target drives both the initiation of Gla-300 and its sustained use beyond 12 months.
This non-interventional trial was designed to investigate the effectiveness of Gla-300-based BBT (administered together with insulin glulisine, as mealtime insulin). The included patients had to meet the Hungarian reimbursement rules, i.e., were switched from human BBT in all cases.
The included patients with the mean age of 60.9 years and mean diabetes duration of more than 10 years at baseline well reflected the typical diabetes population physicians are faced with during their daily practice. According to the indication for switching, the study patients were heterogenic: more than two-third were switched because of inadequate glycemic control, 17.5% because of hypoglycemic events, and the remaining with dual indication.
All glycemic variables collected during the follow-up had decreased significantly by 3 months. Additional statistically significant decrease between 3 and 6 months was detected only in case of HbA1c. At the time of switching from human to analogue regimen, the basal-bolus ratio changed. With the analogue regimen, patients were prescribed higher basal and lower mealtime insulin daily doses which might be predominantly explained by the differences of PK/PD characteristics of the bolus insulins (human regular vs rapid-acting analogue). During the study, change in mean dose of the prandial insulin can be considered as clinically negligible, while the mean dose of BI was increased significantly by about 12% and 19% (by 3 and 6 months compared to the baseline doses, respectively). Although there was a modest body weight reduction during the 6-month follow-up, its magnitude is likely clinically non-significant.
The external validity of observational studies like Toujeo-6M is relatively high as a result of the lack of strict inclusion and exclusion criteria to involve patients. However, it is important to put the results into context with outcomes generated in randomized trials which investigated similar patient populations using the same therapeutic regimen under strict control. Such comparisons can validate the observed findings and vice versa. From the EDITION program, the EDITION 1 trial [5] investigated the same treatment regimen (BBT) similarly in patients with T2DM. Both the baseline glycemic control and the improvement during the 6-month follow-up in our study were higher compared with the results from EDITION 1 (8.9% vs 8.2%, and − 1.4% vs − 0.8%, respectively). It is important to highlight that the final HbA1c values were comparable (7.5% and 7.3% in our study and in EDITION 1, respectively) which indicates that with treatment optimization with Gla-300-based BBT, similar glycemic control can be achieved in daily practice as in strictly controlled protocol-driven randomized controlled trials. Although the daily basal and prandial insulin doses in EDITION 1 were considerably higher than in our study, the difference can be attributed to the inclusion criteria in EDITION 1 which only recruited patients using at least 42 U of BI daily in the trial. Recent results from DELIVER 2 [13] indicate a substantially smaller improvement of HbA1c (− 0.5%) in patients with T2DM who were switched to Gla-300-based insulin regimens (basal-oral or basal-bolus) despite the similar baseline HbA1c value (8.95%) compared to our study. However, there is no information on the dose titration after switching in DELIVER 2, so it cannot be evaluated whether sufficient BI titration has been applied in the study. Furthermore, in DELIVER 2, patients were switched from first-generation basal analogues (insulin glargine U100/mL or insulin detemir) as opposed to our study in which patients were using human insulin-based BBT before switching.
It is encouraging that by the end of the 6-month observational period, more than three-quarters of the patients achieved the HbA1c level below 8%, the threshold defined by the payer to remain on analogue-based insulin regimen with 100% reimbursement beyond 12 months’ use. Interestingly, individual target values were achieved by much lower proportions of the patients which suggests that investigators might have specified too ambitious targets for this challenging patient population with advanced T2DM.
Regarding hypoglycemia, cumulative incidence and event rate of non-severe hypoglycemic events in our study (27.8% and 2.3 events/patient-year) were much lower than those reported in EDITION 1 [5]—a phenomenon that can probably be traced back to the differences in the study design. The frequency of patient–physician contact (in every third month) did not allow as accurate adverse event reports (reporting/recall bias) as the randomized clinical trial did. However, as this supposed bias in hypoglycemic event reporting influenced the periods before and after the inclusion in our study, the conclusion on the beneficial hypoglycemia effects of analogue switching should remain the same.
One of the most important findings in our study relates to severe hypoglycemia, the type of event for which reporting/recall bias is much less relevant. Whilst more than 18 patients (8.3%) reported such an event during the 3-month long pre-study period, only two patients (1.0%) had an episode in the second 3 months of our study period. One should bear in mind that our study population did not exclude patients at high risk of hypoglycemia (which is perhaps reflected in the relatively high proportion of patients reporting such events during the pre-study period being treated with human insulin); thus, it is very encouraging to see such marked reduction after switching. This finding further confirms that severe hypoglycemia risk reduction might be achieved with Gla-300-based insulin regimen in real-world clinical practice, an important result first suggested by DELIVER 2 which showed that Gla-300 had fewer hypoglycemia events associated with hospitalization or emergency department encounters.
The beneficial effect on HbA1c levels was even more prominent in the subgroup switched because of inadequate glycemic control. At the same time the improvement in HbA1c was not significant in the hypo subgroup, a finding which can be explained on the basis of the already well-controlled baseline HbA1c level in this group. Changes in body weight as well as basal and prandial insulin dose in both groups actually showed the same trend that could be seen in the whole population analyses, although in the uncontrolled subgroup both the baseline insulin doses and the increase during the study were considerably higher.
The baseline HbA1c values and hypoglycemic incidence/event rates well reflected the switching indications in the respective patient subgroups. The baseline HbA1c values were significantly lower in the subgroup switched because of hypoglycemic events and did not change significantly over the course of our study, while hypoglycemic event rates decreased to comparable level observed in those switched because of inadequate control.
It is also important to highlight that in the uncontrolled subgroup, the risk of low blood sugar events did not increase parallel with the considerable improvement of their glycemic control. In fact, during the first 3 months of Gla-300 treatment, the hypo risk was reduced compared to the 3-month period before switching. This observation (markedly reduced hypoglycemia risk during first 3 months, a period when 62% of the basal up-titration occurred) was also detected in the entire study population.
The most important limitations of this study can be traced back to its non-interventional design: (i) 17.5% of patients could not be involved in the efficacy analyses because of missing data, and (ii) the accuracy of the detection of hypoglycemic event probably did not meet the demands of a clinical trial as a result of the typical 3-month patient–physician contact frequency.
The most important strength of our non-interventional study is that it demonstrates the beneficial efficacy and safety effects of Gla-300 in patients either inadequately controlled or who experienced a hypoglycemic event.
Conclusions
Gla-300-based analogue BBT in patients switched from human insulin BBT either because of inadequate glycemic control or hypoglycemic events proved to be efficient both in terms of improved glycemic control (in the inadequately controlled subgroup) and decreased risk of hypoglycemia (in both subgroups).
References
Davies MJ, D’Alessio DA, Fradkin J, et al. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;2018(61):2461–98. https://doi.org/10.1007/s00125-018-4729-5.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364–79. https://doi.org/10.2337/dc12-0413.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9. https://doi.org/10.2337/dc14-2441.
Hilgenfeld R, Seipke G, Berchtold H. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs. 2014;74:911–27. https://doi.org/10.1007/s40265-014-0226-4.
Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37:2755–62. https://doi.org/10.2337/dc14-0991.
Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37:3235–43. https://doi.org/10.2337/dc14-0990.
Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/mL compared with glargine 100 U/mL in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17:386–94. https://doi.org/10.1111/dom.12438.
Becker RHA, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units mL-1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL-1. Diabetes Care. 2015;38(4):637–43. https://doi.org/10.2337/dc14-0006.
Mauricio D, Hramiak I. Second-generation insulin analogues—a review of recent real-world data and forthcoming head-to-head comparisons. European Endocrinology. 2018;14(Suppl 1):2–9. https://doi.org/10.17925/EE.2018.14supp1.2.
Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–67. https://doi.org/10.1111/dom.12485.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20(3):541–8. https://doi.org/10.1111/dom.13105.
Pettus J, Roussel R, Zhou FL, et al. Rates of hypoglycemia predicted in patients with type 2 diabetes on insulin glargine 300 U/mL versus first- and second-generation basal insulin analogs: The Real-World LIGHTNING Study. Diabetes Ther. 2019;10:617–33.
Zhou FL, Ye F, Berhanu P, et al. Real-world evidence on clinical and economic outcomes of switching to insulin glargine 300 Units/mL vs other basal insulins in patients with type 2 diabetes on basal insulin. Diabetes Obes Metab. 2018;20:1293–7. https://doi.org/10.1111/dom.13199.
Acknowledgements
The authors wish to thank for all investigators who participated in the study: Ildikó Ádám, MD, Anna Ambrusics, MD, Tímea Baló, MD, Zoltán Balogh, MD, Beáta Bódis, MD, Gyöngyi Csécsei, MD, László Deák, MD, Enikő Dezső, MD, Edit Dobó, MD, István Dobó, MD, Zsolt Domboróczki, MD, Zita Drenyovszki, MD, Gergő Duray, MD, Gyula Fazekas, MD, Andrea Filó, MD, István Fodor, MD, Ágnes Fulcz, MD, András Gáll, MD, Judit Hegedűs, MD, Csilla Kádár, MD, Miklós Káplár, MD, Zsuzsanna Kazi, MD, János Kis, MD, Irén Kovács, MD, Margit Lukács, MD, Éva Mihály, MD, István Móricz, MD, Judit Nádas, MD, István Páll, MD, Zsuzsanna Papp, MD, Béla Polocsányi, MD, Krisztián Sepp, MD, Judit Szabó, MD, Sándor Szabó, MD, Piroska Szakács, MD, Enikő Szfárli, MD, Erzsébet Szilágyi, MD, József Takács, MD, Erzsébet Thaisz, MD, Mária Tóth, MD, Viktor Vass, MD, Tibor Végh, MD, Klára Vida, MD. The authors also wish to thank the participants of the study.
Funding
Sanofi Hungary was the sponsor of the study. The study did not receive any additional grant or funding. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. The study sponsor is funding the journal fees.
Medical Writing Assistance
Assistance with submission of the manuscript was provided by Clemence Hindley PhD of Fishawack Communications Ltd. and funded by Sanofi.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Tibor Hidvégi: planning and designing the study, planning the data analysis. Zoltán Balogh: participating in the study and planning the publication. Viktor Vass: participating in the study and planning the publication. Gábor Kovács: planning and designing the study, planning and performing the data analysis. Péter Stella: supervising and performing the data analysis.
Disclosures
Tibor Hidvégi had a contract and received remuneration from Sanofi Hungary for participating in the study. Zoltán Balogh had a contract and received remuneration from Sanofi Hungary for participating in the study. Viktor Vass had a contract and received remuneration from Sanofi Hungary for participating in the study. Gábor Kovács had a contract and received remuneration from Sanofi Hungary for participating in the study. Peter Stella is an employee of Sanofi Hungary.
Compliance with Ethics Guidelines
The study (named “Toujeo-6M”) conformed to the Helsinki Declaration of 1964, as revised in 2013, and was approved by Hungarian National Institute of Pharmacy and Nutrition (registration code OGYI/44755-7/2015) and received national ethical approval as well. All patients gave written informed consent before any data collection.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11323673.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hidvégi, T., Balogh, Z., Vass, V. et al. Insulin Glargine 300 U/mL and Insulin Glulisine Treatment in Patients with Type 2 Diabetes: A Non-Interventional Study of Effectiveness in Routine Clinical Practice. Diabetes Ther 11, 467–478 (2020). https://doi.org/10.1007/s13300-019-00746-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-00746-4