Abstract
Introduction
The aim of the present study was to use real-world data from Swedish primary-care and national registries to understand clinical outcomes in patients with Type 2 diabetes (T2D) treated with liraglutide in clinical practice, and to compare with data from those treated with sitagliptin.
Methods
This was a non-interventional, retrospective study conducted between February 2014 and September 2014 using T2D patient data from Swedish primary-care centers and national healthcare registries. The primary objective was to assess the effectiveness of liraglutide in control of glycemia and body weight in clinical practice (stage 1). The secondary objective was to compare the clinical effectiveness of liraglutide with sitagliptin on glycemic control and body weight in clinical practice in a propensity-score-matched population (stage 2).
Results
In stage 1 (n = 402), 39.4% of patients treated with liraglutide achieved ≥1.0% (10.9 mmol/mol) reduction in glycated hemoglobin (HbA1c) after 180 days of treatment and 54.9% achieved the target HbA1c of <7.0% (53.0 mmol/mol). Moreover, compared with baseline, 22.5% of patients treated with liraglutide achieved both ≥1.0% reduction in HbA1c and ≥3.0% reduction in body weight. In stage 2, a significantly greater proportion of patients receiving liraglutide (n = 180) than sitagliptin (n = 208) achieved ≥1.0% reduction in HbA1c [52.9% vs 33.5%, respectively (P = 0.0002)]. Mean body-weight loss was also significantly greater in patients receiving liraglutide vs sitagliptin [−3.5 vs −1.3 kg, respectively (P < 0.0001)].
Conclusion
This study provides real-world evidence from Sweden corroborating previous clinical trials that demonstrate greater efficacy of liraglutide over sitagliptin on glycemic control and body-weight reduction in patients with T2D.
Funding
Novo Nordisk A/S.
Trial registration
ClinicalTrials.gov identifier NCT02077946.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Good glycemic control is a cornerstone of prevention of long-term diabetic complications and reduces mortality in patients with type 2 diabetes (T2D) [1–3]. Incretin-based therapies, such as glucagon-like peptide-1 receptor agonists (GLP-1RAs) or dipeptidyl peptidase-4 inhibitors (DPP-4is), are based on the intestinal hormone GLP-1 and lead to improved glycemic control and have a beneficial weight effect in patients with T2D [4].
Liraglutide is a once-daily human GLP-1RA approved in over 100 countries worldwide for the treatment of adults with T2D by once-daily subcutaneous administration [5]. In the European Union (EU)/Sweden, liraglutide at doses up to 1.8 mg is indicated (approved in January 2010) for treatment of adults with T2D to achieve glycemic control in combination with oral glucose-lowering drugs when these, together with diet and exercise, provide inadequate glycemic control. In April 2014, liraglutide in T2D was approved in EU/Sweden for use in combination with basal insulin.
Sitagliptin, a DPP-4i approved for use in Sweden in June 2007, both as monotherapy and in combination with other oral glucose-lowering drugs and insulin for the treatment of T2D, has been shown to improve glycemic control in patients with T2D in several clinical trials [6, 7]. Moreover, sitagliptin is currently the most commonly prescribed DPP-4i in Sweden.
Data from clinical trials demonstrated that liraglutide was superior to sitagliptin in terms of reducing both glycated hemoglobin (HbA1c) and body weight in patients with T2D [8, 9].
Randomized controlled trials (RCTs) are the gold standard for evaluating efficacy and safety of therapeutic interventions [10], but regulatory agencies and payers worldwide require assurance that RCT findings translate into clinical benefits in the broader patient populations of clinical practice. Healthcare providers, moreover, increasingly look for real-world clinical evidence that new therapies provide better outcomes and/or greater value than existing standards of care [11].
The primary objective of this study was to assess the effectiveness of liraglutide in control of glycemia and body weight in clinical practice in Sweden. The secondary objective was to compare the effectiveness of liraglutide vs sitagliptin on glycemic control and body-weight reduction. To reduce the possible confounding effect on comparison of the two treatments by different baseline patient characteristics, which naturally vary more in observational studies than in randomized trials, propensity score-matching (PSM) was applied.
Methods
Study Design and Data Extraction
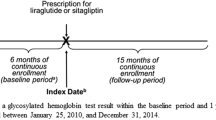
This non-interventional, retrospective study was conducted between February 2014 and September 2014 where data from patients with T2D were collected from primary care centers (PCCs) and national healthcare registries in Sweden. Fifty-three PCCs were selected from a large geographical area of Sweden, representing ~5–6% of the Swedish population. Data were extracted from electronic medical records (EMRs) held by PCCs using the Pygargus Customised eXtraction Program, CXP 3.0 (Pygargus; Stockholm, Sweden), a validated data-extraction tool [12]. Primary care medical records including laboratory measurements were linked with data from the national patient registry, national prescription registry, and national LISA (longitudinal integration database for health insurance and labor market studies) database kept at Statistics Sweden, for 2010–2013. Data on diabetes and comorbidities were also collected from the EMRs from 1996 onwards if available for each patient.
Patients
Inclusion criteria comprised the following: patients treated in primary care with a diagnosis of diabetes (International Classification of Diseases 10th edition codes; E10, E11, E13, E14) and receiving treatment with liraglutide [Anatomical Therapeutic Chemical (ATC)-code A10BX07] or sitagliptin (ATC-code A10BH01) who were judged to have T2D, age ≥18 years, treatment with liraglutide or sitagliptin started between January 2010 and December 2013 (date of first prescription purchase = index date), a minimum of 180 days’ history within the database before their index date, and at least one HbA1c and body-weight measurement after 180 days’ therapy (defined as 180 days −60/+180 days, always choosing the observation closest to 180 days). Exclusion criteria comprised the following: current or previous cancer; end-stage liver disease; end-stage renal failure; history of gestational diabetes; or previous GLP-1RA or DPP-4i use other than described in the inclusion criteria.
Outcome Measures
Outcomes data were measured ~180 days after index date and compared with data at or before index date (i.e. baseline, which was the period of 90 days before index date and 6 days thereafter, always choosing the observation closest to index date). Similar endpoints were used in both stages of the study (Table S1). Information on prescribed doses of liraglutide or sitagliptin was collected for all patients, and adherence to prescribed therapy was calculated for each patient and then summarized by treatment group. Adherence to therapy during the study period was determined using the medication possession ratio (MPR) (adherence threshold; ≥80%).
Statistical Analyses
A feasibility assessment was conducted at 10 PCCs to determine sample size for the present study. At least 750 patients prescribed liraglutide were needed for 80% power to find a statistically significant difference between treatment groups at the 5% level in proportion of patients achieving ≥1.0% reduction in HbA1c (primary endpoint). The number of patients required was based on observations that 40% and 28% of patients receiving liraglutide and sitagliptin, respectively, reached the primary endpoint in the feasibility study and only 44% had HbA1c measurements.
All statistical analyses were performed using SAS statistical software, SAS version 9.3 (Cary, NC, USA). Descriptive statistics were calculated for all variables. In stage 1, changes during the study period in the primary endpoint were analyzed using a Chi squared test. Changes in HbA1c and body weight were compared with baseline using t tests for the overall population and the outcome, e.g. change in HbA1c and body weight were compared using ANCOVA models with the baseline values of HbA1c and body weight, respectively, as covariates to compensate for the expected difference in these variables at baseline. Other variables [e.g. low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides] were also compared using an ANCOVA with baseline value of the variable as covariate. The predictors for achieving HbA1c <7.0% were investigated using logistic regression.
In stage 2, propensity scores were first calculated from 29 pre-defined variables covering demography, general disease and diabetes (with values calculated on or before index date). The liraglutide group was then matched with the sitagliptin group using a ‘greedy’ five-digit-matching without replacement and with best match retained at each step. A pilot survey of the available data from this study showed that liraglutide-treated patients had higher baseline body weight, body mass index (BMI), and HbA1c than sitagliptin-treated patients. Therefore, these variables were excluded from the PSM to avoid selecting patients from the tails of the body weight, BMI and HbA1c distributions. Instead these variables were included as covariates. The study results were expected to be confounded by changes in insulin dose during the 180-day treatment period. Therefore, patients were excluded from the main analysis population if the insulin dose had changed during this time. These patients were included in a post hoc analysis, whereby change in HbA1c and body weight were investigated stratified by change in insulin dose with the baseline variable as covariates. In stage 2, treatment groups were compared using Chi squared tests for categorical variables and ANCOVAs for continuous variables.
Four pre-planned sensitivity analyses were undertaken to investigate the robustness relating to the primary endpoint in stage 2. In the analyses, variables were incorporated singly into the matching process in a cumulative approach (analysis #1); each variable was added into the matching procedure and then removed in turn until all variables had been examined (analysis #2), and the propensity scores were divided into quartiles after matching (analysis #3). The final sensitivity analysis was conducted for all patients (analysis #4).
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practices. Study approval was obtained from the Ethical Review Board in Stockholm, Sweden, and approval for access to EMRs from individual PCCs. National data-protection regulations were observed throughout, but patient-informed consent was not required due to the de-identified nature of collected data. The study is registered at ClinicalTrials.gov (NCT02077946).
Results
Patient Disposition and Treatment
In total, data were extracted from EMRs for 3676 patients (Fig. 1). Of these, 1155 patients were prescribed liraglutide (1.2 mg prescribed for 89% of patients) and 2521 were prescribed sitagliptin (100 mg prescribed for 95% of patients) (Figure S1). A total of 402 patients prescribed liraglutide (88% of patients with MPR ≥80%) were included in stage 1.
In the liraglutide group, mean insulin dose for all patients treated with insulin before (34.3% of patients) and after (30.3% of patients) index date was 66 and 54 IU, respectively (stage 1; n = 402). By comparison, mean insulin dose for all patients treated with insulin before (18.1% of patients) and after (19.2% of patients) index date was 79 and 74 IU, respectively, in the sitagliptin group (n = 657). Approximately 87% of patients prescribed liraglutide and 80% of patients prescribed sitagliptin had a final HbA1c measurement within 80 days of discontinuing their respective treatment (Figure S2).
After PSM, there were 317 patients in each treatment group. Stage 2, however, comprised 180 patients receiving liraglutide and 208 patients receiving sitagliptin [MPR ≥80%; 63.3% vs 52.9%, respectively (P = 0.038)] due to the exclusion of patients with a change in insulin dose after index date.
Patient Characteristics at Index Date
Before PSM and using sitagliptin as a reference, patients treated with liraglutide received their first diabetes diagnosis at a younger age, were younger, heavier, and had a higher BMI (Table 1). Furthermore, liraglutide-treated patients had a greater disease severity, as evidenced by a higher baseline HbA1c and a greater number of prescriptions of insulin in the 2 years before index date. Sitagliptin-treated patients were often sulfonylurea (SU) users before index date [mean (standard deviation) prescriptions/patient/year 0.9 ± 1.8 in liraglutide-treated patients vs 1.3 ± 3.2 for sitagliptin-treated patients, P = 0.012] (Table S2). After PSM, body weight and BMI (not included in the matching) remained significantly different between the groups. No other parameters differed between the groups at index date after PSM.
Clinical Effectiveness of Liraglutide (Stage 1)
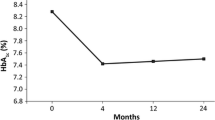
In total, 39.4% of the 402 patients treated with liraglutide achieved ≥1.0% (≥10.9 mmol/mol) reduction in HbA1c after 180 days’ treatment (primary endpoint) and 54.9% achieved the HbA1c target of <7.0% [<53.0 mmol/mol (Table 2)]. Baseline HbA1c was the only predictor of achieving an end-of-study HbA1c value <7.0% (<53.0 mmol/mol) that was statistically significant (P < 0.0001). HbA1c and body weight were significantly reduced from baseline after 180 days’ treatment with liraglutide and 22.5% of patients treated with liraglutide achieved the composite endpoint based on the National Institute for Health and Care Excellence (NICE) guidelines for liraglutide treatment, i.e. both a reduction of ≥1.0% (≥10.9 mmol/mol) in HbA1c and ≥3.0% of initial body weight (Table 2) [13]. Subgroup analyses showed larger decreases in HbA1c for patients with a higher vs lower baseline HbA1c (P < 0.0001), shorter vs longer duration of diabetes before index date (P = 0.0384), and with a less vs more intensive glucose-lowering treatment before index date (based on several subgroup analyses, all with P < 0.0497). There were no statistically significant associations between change in HbA1c and baseline body weight or BMI categories (P = 0.1058 and P = 0.9368, respectively). A statistically significant reduction in total cholesterol and systolic blood pressure (BP) after 180 days’ treatment with liraglutide were found (Table 2). However, no significant effects were observed on LDL-cholesterol, HDL-cholesterol, triglycerides, creatinine or diastolic BP (Table 2).
Effectiveness of Liraglutide vs Sitagliptin (Stage 2)
After 180 days’ treatment, 52.9% of patients receiving liraglutide achieved ≥1.0% (≥10.9 mmol/mol) reduction in HbA1c vs 33.5% of patients treated with sitagliptin (P = 0.0002) (Fig. 2). Results from three of the pre-specified sensitivity analyses showed similar results. In sensitivity analysis #3, where the population was divided into quartiles based on the propensity score value, only the highest quartile demonstrated significance. Patients in this quartile are characterized by being older and using less insulin.
Patients achieving the primary endpoint (≥1.0% reduction in HbA1c), a reduction of ≥3.0% of initial body-weight, and fulfilling NICE criteria (for GLP-1RA treatment) (composite endpoint—a reduction ≥1.0% in HbA1c and ≥3.0% of initial body-weight) after 180 days of treatment. Data are for cohorts after PSM and after the exclusion of patients with a change in insulin dose during the treatment period. Proportions are calculated from patients with available data. GLP-1RA glucagon-like peptide-1 receptor agonist, HbA1c glycated hemoglobin, n number of patients, NICE National Institute for Health and Care Excellence, PSM propensity score matching
In total, 71.1% of patients receiving liraglutide achieved the HbA1c target of <7.0% (<53.0 mmol/mol) compared with 63.5% receiving sitagliptin (P = 0.1105). Stratifying this analysis based on baseline HbA1c (post hoc analysis) demonstrated that in patients who had a baseline HbA1c ≥7.0% (≥53.0 mmol/mol), optimal glycemic control [HbA1c <7.0% (<53.0 mmol/mol)] at study-end was achieved by 39.4% of patients treated with liraglutide and 24.5% of patients treated with sitagliptin. The post hoc analysis demonstrated that there was a higher proportion of patients treated with sitagliptin (6.0%) that increased their baseline HbA1c from <7.0% (<53.0 mmol/mol) to ≥7.0% (≥53.0 mmol/mol) at study-end compared with patients treated with liraglutide (1.5%). These findings are despite approximately one-third of patients in each group having both a baseline and study-end HbA1c <7.0% (<53.0 mmol/mol), (liraglutide, 32.6%; sitagliptin, 36.5%). There were significantly greater HbA1c and body-weight reductions in patients receiving liraglutide compared with sitagliptin [HbA1c: −1.2% (−13.1 mmol/mol) vs –0.7% (−7.7 mmol/mol); body-weight: −3.5 vs −1.4 kg, respectively] (Fig. 3). Consequently, a significantly greater proportion of the liraglutide group than the sitagliptin group achieved ≥3.0% reduction in baseline body weight (51.6% vs 26.0%, respectively) and the composite endpoint based on NICE guidelines for liraglutide treatment (27.0% vs 9.6%, respectively) (Fig. 2) [13]. No statistically significant difference between the groups was found for change from baseline in LDL-cholesterol, HDL-cholesterol, total cholesterol, BP (systolic and diastolic), and creatinine. There was, however, a significantly greater reduction in triglycerides observed with patients treated with sitagliptin vs liraglutide [−0.5 vs −0.1 mmol/L, respectively (P = 0.0093)].
Mean changes in HbA1c and body weight after 180 days of treatment. Data are for cohorts after PSM and after the exclusion of patients with a change in insulin dose during the treatment period. Mean values are calculated from patients with available data. HbA1c glycated hemoglobin, n number of patients, PSM propensity score matching
Post hoc Analysis Including Patients with Changes in Insulin Dose
After PSM, there were 317 patients in each treatment group. Of the 137 (43.2%) patients excluded from stage 2 analysis in the liraglutide group due to changes in insulin dose, 101 (73.7%) had a mean reduction in insulin dose of −33 IU (−25.4%) and 36 (26.3%) had a mean increase of 30 IU (23.1%). Of the 109 (34.4%) patients excluded in the sitagliptin group, 56 (51.4%) had a mean reduction in insulin dose of 31 IU (−23.0%), and 53 (48.6%) had a mean increase of 32 IU (23.8%).
Of those patients that had a decrease in insulin dose, mean reductions in HbA1c at the end of treatment were −0.23% (−2.5 mmol/mol) vs −0.27% (−3.0 mmol/mol) in the liraglutide and sitagliptin groups, respectively (P = 0.5889). Of those that had an increase in insulin dose, mean reductions in HbA1c at the end of treatment were −1.19% (−13.0 mmol/mol) vs −0.69% (−7.5 mmol/mol) in the liraglutide and sitagliptin groups, respectively (P = 0.1324). Of those patients that had a decrease in insulin dose, mean reductions in body weight at the end of treatment were −4.5 vs −1.8 kg in the liraglutide and sitagliptin groups, respectively (P = 0.0173). Of those that had an increase in insulin dose, mean changes in body weight at the end of treatment were −1.1 vs 0.4 kg in the liraglutide and sitagliptin groups, respectively (P = 0.0446).
Discussion
In the current study, more than one-third of patients treated with liraglutide achieved the goal of ≥1.0% (≥10.9 mmol/mol) reduction in HbA1c. Furthermore, over half of patients receiving liraglutide achieved the HbA1c target of <7.0% (<53.0 mmol/mol) and there were greater reductions in HbA1c and body weight in patients receiving liraglutide than in those receiving sitagliptin.
Using a retrospective cohort study design allowed for collection and analysis of readily available data from EMRs, and the application of PSM limits the effects of confounding inherent in the design. The results from this study may be considered representative of the national profile of patients with T2D in Sweden, in part due to availability of data up to as recently as May 2014 and the multicenter design. However, due to the procedure of PSM and the observational nature of this study, selection bias cannot be excluded. Additionally, the lack of safety/tolerability evaluation is a limitation of the study as it does not provide a full overview of the benefit/risk profile of the drugs.
In the present study, a similar proportion of the liraglutide group and the sitagliptin group were excluded. However, there was a noticeable difference between treatment groups in the proportion of patients previously treated with GLP-1RAs and DPP-4is (higher in the sitagliptin group). This finding may, in part, be explained by the higher age of the patients receiving sitagliptin and the fact that more patients were on insulin before index date in the liraglutide group, pointing toward a more severe disease cause in patients treated with liraglutide.
As intended for observational studies, the inclusion and exclusion criteria used in the current study were not as strict as those of clinical trials. Therefore, the normal clinical practice and the prescribing habits of physicians in Sweden could be investigated. Results generated before applying PSM demonstrated that clinicians in Sweden elect to prescribe liraglutide to patients with T2D who are younger and heavier with a greater disease severity. This is comparable with recent findings from a retrospective study from the UK [14]. In the current study, patients in the liraglutide group had a higher mean baseline body weight than those in the sitagliptin group. Therefore, physicians appear not to select SUs for patients in the liraglutide group to prevent further weight gain, which is a known side-effect with SU treatment [15]. After performing PSM, 137 and 109 patients in the liraglutide and sitagliptin groups, respectively, were excluded from the study because of potential confounding caused by changes in insulin dose within the 180 days’ observational period. Before April 2014, use of liraglutide in combination with basal insulin was considered ‘off-label’; however, the fact that such a high proportion of patients were receiving liraglutide with basal insulin at that time may indicate that many physicians in Sweden were seeking better glycemic control for this group of patients. Recently, an RCT showed beneficial effects of liraglutide in patients with T2D treated with multiple daily insulin injections [16]. Data from the post hoc analysis of the current study demonstrated that when physicians in Sweden added liraglutide to basal insulin, they tended to reduce the insulin dose. As the use of basal insulin with liraglutide was considered ‘off-label’ at this time, it is possible that many physicians reduced the insulin dose more than was necessary due to safety concerns. Also noteworthy in the study, the likelihood that a patient adhered to the treatment during follow-up was higher with the liraglutide regimen than with sitagliptin, as demonstrated by the greater proportion of patients with an MPR ≥80% in the liraglutide PSM population. This latter finding may reflect greater treatment satisfaction and/or better glycemic control in patients receiving liraglutide than those receiving sitagliptin [17].
In stage 1, the proportion of patients achieving a ≥1.0% (≥10.9 mmol/mol) reduction in HbA1c was comparable with a former meta-analysis (47.0% [18] and real-world (29.3% [14]) data. Furthermore, the mean change in HbA1c from baseline after 180 days’ treatment reported with liraglutide in the current study was similar to previous findings from other real-world studies [−0.6% (−6.5 mmol/mol) to −1.1% (−11.8 mmol/mol)] [14, 19–22]. The association between greater reductions in HbA1c in response to liraglutide in patients with high baseline HbA1c, a short diabetes duration, and less intensive glucose-lowering treatment regimens reported in the current study also are comparable with previous findings [23–26]. However, confounding caused by greater changes in insulin dose in patients with a longer diabetes duration may render the association with HbA1c reduction difficult to interpret. Low baseline HbA1c was identified as a predictor of achieving glycemic control, which is consistent with findings from a recent meta-analysis of 25 RCTs [18]. The mean change in body weight from baseline after 180 days’ treatment reported with liraglutide in the current study was similar to previous findings from another real-world study (−3.8 kg [14]) and a recent RCT (−4.5 kg [27]).
In stage 2, the change in HbA1c from baseline in response to liraglutide and sitagliptin was evaluated using three different approaches: HbA1c reduction of ≥1.0% (≥10.9 mmol/mol); absolute change in HbA1c; and end-of-study value of HbA1c <7.0% (<53.0 mmol/mol). In all of these analyses, results for liraglutide were significantly better than those for sitagliptin. These results, and the finding that body-weight reduction with liraglutide was also greater than with sitagliptin, are largely in agreement with results obtained in earlier prospective RCTs [8, 9, 28] and retrospective studies [14, 29]. In stage 2, findings relating to the primary endpoint were verified by three sensitivity analyses. The non-significant findings with the fourth sensitivity analysis may be due to the low numbers of patients in each sub-population as the patients receiving liraglutide in each sub-population had a higher percentage of patients with ≥1.0% (≥10.9 mmol/mol) reduction in HbA1c than those receiving sitagliptin. The findings relating to body-weight reduction were not unexpected as sitagliptin is generally considered to be body-weight neutral [30]. It should be noted that baseline body weight and BMI were not included in the PSM and although baseline body weight was adjusted for in the analyses, results related to body weight may be interpreted with greater caution than those related to HbA1c. There was a significantly greater reduction in triglycerides in the sitagliptin group compared to the liraglutide group. These findings are in contrast to previous studies which reported no difference in triglyceride levels between the liraglutide and sitagliptin groups at either 26 or 52 weeks [8, 9].
Conclusion
This study provides real-world evidence that glycemic control improves and body weight is reduced after initiating liraglutide in patients with T2D in primary care in Sweden. It further demonstrates greater effectiveness of liraglutide over sitagliptin on glycemic control and body-weight reduction in clinical practice in patients with T2D, corroborating findings in previous head-to-head clinical trials.
References
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015;373:1720–32.
Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16.
Novo Nordisk. Victoza summary of product characteristics. 2015; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed 29 Feb 2016.
Ahren B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007;30:1344–50.
Ahren B. Clinical results of treating type 2 diabetic patients with sitagliptin, vildagliptin or saxagliptin—diabetes control and potential adverse events. Best Pract Res Clin Endocrinol Metab. 2009;23:487–98.
Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56.
Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397–407.
Akobeng AK. Understanding randomised controlled trials. Arch Dis Child. 2005;90:840–4.
Zueger PM, Schultz NM, Lee TA. Cost effectiveness of liraglutide in type II diabetes: a systematic review. Pharmacoeconomics. 2014;32:1079–91.
Martinell M, Stalhammar J, Hallqvist J. Automated data extraction—a feasible way to construct patient registers of primary care utilization. Ups J Med Sci. 2012;117:52–6.
National Institute for Health and Care Excellence. Liraglutide for the treatment of type 2 diabetes mellitus. 2014. http://www.nice.org.uk/guidance/ng28. Accessed 3 March 2016.
Nyeland ME, Ploug UJ, Richards A, et al. Evaluation of the effectiveness of liraglutide and sitagliptin in type 2 diabetes: a retrospective study in UK primary care. Int J Clin Pract. 2015;69:281–91.
Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–72.
Lind M, Hirsch IB, Tuomilehto J, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI Liraglutide trial). BMJ. 2015;351:h5364.
Hendrychova T, Vytrisalova M, Smahelova A, Vlcek J, Kubena AA. Adherence in adults with type 1 diabetes mellitus correlates with treatment satisfaction but not with adverse events. Patient Prefer Adherence. 2013;7:867–76.
Esposito K, Mosca C, Brancario C, Chiodini P, Ceriello A, Giugliano D. GLP-1 receptor agonists and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Curr Med Res Opin. 2011;27:1519–28.
Lee WC, Dekoven M, Bouchard J, Massoudi M, Langer J. Improved real-world glycaemic outcomes with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes Metab. 2014;16:819–26.
Lipowsky C, Sze L, Krull I, Brandle M. Liraglutide as add-on therapy to insulin in type 2 diabetes mellitus: a retrospective, observational study from a daily clinical practice setting in Switzerland. Diabetes Ther. 2015;6:41–7.
Bashier AM, Hussain AA, Abdelgadir EI, et al. Liraglutide effect in reducing HbA1c and weight in Arab population with type 2 diabetes, a prospective observational trial. J Diabetes Metab Disord. 2015;14:48.
Yokoyama H, Hirao K, Yamaguchi K, et al. Liraglutide versus sitagliptin in a 24-week, multicenter, open-label, randomized, parallel-group study in Japanese type 2 diabetes mellitus patients responding inadequately to a sulfonylurea and/or one or two other oral antidiabetic drugs (JDDM 33). Jpn Clin Med. 2014;5:33–41.
Deacon CF, Mannucci E, Ahren B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab. 2012;14:762–7.
Kozawa J, Inoue K, Iwamoto R, et al. Liraglutide is effective in type 2 diabetic patients with sustained endogenous insulin-secreting capacity. J Diabetes Investig. 2012;3:294–7.
Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268–78.
Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90.
de Wit HM, Vervoort GM, Jansen HJ, de Grauw WJ, de Galan BE, Tack CJ. Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia. 2014;57:1812–9.
Scheen AJ. DPP-4 inhibitors in the management of type 2 diabetes: a critical review of head-to-head trials. Diabetes Metab. 2012;38:89–101.
Evans M, McEwan P, O’Shea R, George L. A retrospective, case-note survey of type 2 diabetes patients prescribed incretin-based therapies in clinical practice. Diabetes Ther. 2013;4:27–40.
Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–7.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novo Nordisk A/S, Bagsvaerd, Denmark. The authors would like to thank all investigators involved in the study and Karl Gledhill, Ph.D., of Watermeadow Medical, UK, an Ashfield Company, for providing medical writing and editorial assistance to the authors during the preparation of this manuscript. Support for this assistance was funded by Novo Nordisk A/S. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author contributions
Study design: Uffe J. Ploug, Ragnar Linder, Gunnar Johansson, Sara Larsen, Helge Gydesen, Marcus Lind. Study contact/data collection: Mozhgan Dorkhan, Irene Svenningsson, Uffe Ploug, Ragnar Linder, Gunnar Johansson, Per-Olov Matsson, Sara Larsen. Data analysis: Mozhgan Dorkhan, Uffe Ploug, Ragnar Linder, Gunnar Johansson, Sara Larsen, Helge Gydesen, Marcus Lind, Leif Jørgensen.
Disclosures
Marcus Lind has been a consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Co, Novo Nordisk, and Sanofi, participated in advisory boards for Novo Nordisk and received research grants from AstraZeneca and Novo Nordisk. Ragnar Linder is a full-time employee of Pygargus AB. Uffe Ploug is a full-time employee of Novo Nordisk A/S. Helge Gydesen is a full-time employee of Novo Nordisk A/S. Mozhgan Dorkhan is a full-time employee of Novo Nordisk A/S. Sara Larsen is a full-time employee of Novo Nordisk A/S. Gunnar Johansson has participated in the steering committee by Novo Nordisk for this study and served on advisory boards arranged by Astra Zeneca and Takeda. Irene Svenningsson, Leif Jørgensen and Per-Olov Matsson have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki and the Guidelines for Good Pharmacoepidemiology Practices. Study approval was obtained from the Ethical Review Board in Stockholm, Sweden, and approval for access to EMRs from individual PCCs. National data-protection regulations were observed throughout, but patient-informed consent was not required due to the de-identified nature of collected data. The study is registered at ClinicalTrials.gov (NCT02077946).
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C1D4F060500DA6D1.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lind, M., Matsson, PO., Linder, R. et al. Clinical Effectiveness of Liraglutide vs Sitagliptin on Glycemic Control and Body Weight in Patients with Type 2 Diabetes: A Retrospective Assessment in Sweden. Diabetes Ther 7, 321–333 (2016). https://doi.org/10.1007/s13300-016-0173-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-016-0173-z