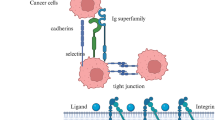
Abstract
A disintegrin and metalloproteinase (ADAM) family members are known to process the target membrane-bound molecules through the quick induction of their protease activities under interaction with other molecules, which have diverse roles in tissue morphogenesis and pathophysiological remodeling. Among these, ADAM17 is a membrane-bound protease that sheds the extracellular domain of various receptors or its ligands from the cell membrane and subsequently activates downstream signaling transduction pathways. Importantly, breast cancer remains a mainspring of cancer-induced death in women, and numerous regulatory pathways have been implicated in the formation of breast cancer. Substantial evidence has demonstrated that an obvious increased in ADAM17 cell surface expression has been discovered in breast cancer and was shown to be associated with mammary tumorigenesis, invasiveness, and drug resistance. Over the last decades, it has received more than its share of attention that ADAM17 plays a potential role in breast cancer, including cell proliferation, invasion, angiogenesis, apoptosis, and trastuzumab resistance. In our review, we discuss the mechanisms through which ADAM17 acts on breast cancer tumorigenesis and progression. Thus, this will provide further impetus for exploiting ADAM17 as a new target for breast cancer treatment.





Similar content being viewed by others
References
Filipova A, Seifrtova M, Mokry J, Dvorak J, Rezacova M, Filip S, et al. Breast cancer and cancer stem cells: a mini-review. Tumori. 2014;100(4):363–9. doi:10.1700/1636.17886.
Diessner J, Bruttel V, Becker K, Pawlik M, Stein R, Hausler S, et al. Targeting breast cancer stem cells with HER2-specific antibodies and natural killer cells. Am J Cancer Res. 2013;3(2):211–20.
Kim K, Chie EK, Han W, Noh DY, Oh DY, Im SA, et al. Prognostic factors affecting the outcome of salvage radiotherapy for isolated locoregional recurrence after mastectomy. Am J Clin Oncol. 2010;33(1):23–7. doi:10.1097/COC.0b013e31819e2c02.
George BP, Abrahamse H. A review on novel breast cancer therapies: photodynamic therapy and plant derived agent induced cell death mechanisms. Anticancer Agents Med Chem. 2016;16:793–801.
McGowan PM, McKiernan E, Bolster F, Ryan BM, Hill AD, McDermott EW, et al. ADAM-17 predicts adverse outcome in patients with breast cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2008;19(6):1075–81. doi:10.1093/annonc/mdm609.
McGowan PM, Ryan BM, Hill AD, McDermott E, O’Higgins N, Duffy MJ. ADAM-17 expression in breast cancer correlates with variables of tumor progression. Clin Cancer Res: An Off J Am Assoc Cancer Res. 2007;13(8):2335–43. doi:10.1158/1078-0432.CCR-06-2092.
Sternlicht MD, Sunnarborg SW. The ADAM17-amphiregulin-EGFR axis in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2008;13(2):181–94. doi:10.1007/s10911-008-9084-6.
Zheng X, Jiang F, Katakowski M, Zhang ZG, Lu QE, Chopp M. ADAM17 promotes breast cancer cell malignant phenotype through EGFR-PI3K-AKT activation. Cancer Biol Ther. 2009;8(11):1045–54.
Duffy MJ, Mullooly M, O’Donovan N, Sukor S, Crown J, Pierce A, et al. The ADAMs family of proteases: new biomarkers and therapeutic targets for cancer? Clin Proteomics. 2011;8(1):9. doi:10.1186/1559-0275-8-9.
Bass R, Edwards DR. ADAMs and protein disulfide isomerase: the key to regulated cell-surface protein ectodomain shedding? Biochem J. 2010;428(3):e3–5. doi:10.1042/BJ20100568.
Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385(6618):733–6. doi:10.1038/385733a0.
Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385(6618):729–33. doi:10.1038/385729a0.
Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011;32(8):380–7. doi:10.1016/j.it.2011.05.005.
Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des. 2009;15(20):2319–35.
Zhou BB, Peyton M, He B, Liu C, Girard L, Caudler E, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in non-small cell lung cancer. Cancer Cell. 2006;10(1):39–50. doi:10.1016/j.ccr.2006.05.024.
Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164(5):769–79. doi:10.1083/jcb.200307137.
Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6(1):32–43. doi:10.1038/nrm1548.
Maretzky T, Evers A, Zhou W, Swendeman SL, Wong PM, Rafii S, et al. Migration of growth factor-stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun. 2011;2:229. doi:10.1038/ncomms1232.
Cai M, Wang Z, Zhang J, Zhou H, Jin L, Bai R, et al. Adam17, a target of Mir-326, promotes Emt-induced cells invasion in lung adenocarcinoma. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol. 2015;36(3):1175–85. doi:10.1159/000430288.
Yamamoto K, Trad A, Baumgart A, Huske L, Lorenzen I, Chalaris A, et al. A novel bispecific single-chain antibody for ADAM17 and CD3 induces T-cell-mediated lysis of prostate cancer cells. Biochem J. 2012;445(1):135–44. doi:10.1042/BJ20120433.
Zheng X, Jiang F, Katakowski M, Lu Y, Chopp M. ADAM17 promotes glioma cell malignant phenotype. Mol Carcinog. 2012;51(2):150–64. doi:10.1002/mc.20772.
Xu Q, Ying M, Chen G, Lin A, Xie Y, Ohara N, et al. ADAM17 is associated with EMMPRIN and predicts poor prognosis in patients with uterine cervical carcinoma. Tumour Biol: J Int Soc Oncodevelopmental Biol Med. 2014;35(8):7575–86. doi:10.1007/s13277-014-1990-1.
Richards FM, Tape CJ, Jodrell DI, Murphy G. Anti-tumour effects of a specific anti-ADAM17 antibody in an ovarian cancer model in vivo. PLoS One. 2012;7(7):e40597. doi:10.1371/journal.pone.0040597.
Blanchot-Jossic F, Jarry A, Masson D, Bach-Ngohou K, Paineau J, Denis MG, et al. Up-regulated expression of ADAM17 in human colon carcinoma: co-expression with EGFR in neoplastic and endothelial cells. J Pathol. 2005;207(2):156–63. doi:10.1002/path.1814.
Ringel J, Jesnowski R, Moniaux N, Luttges J, Ringel J, Choudhury A, et al. Aberrant expression of a disintegrin and metalloproteinase 17/tumor necrosis factor-alpha converting enzyme increases the malignant potential in human pancreatic ductal adenocarcinoma. Cancer Res. 2006;66(18):9045–53. doi:10.1158/0008-5472.CAN-05-3287.
Wu J, Yin L, Jiang N, Guo WJ, Gu JJ, Chen M, et al. MiR-145, a microRNA targeting ADAM17, inhibits the invasion and migration of nasopharyngeal carcinoma cells. Exp Cell Res. 2015;338(2):232–8. doi:10.1016/j.yexcr.2015.08.006.
Aydin D, Bilici A, Yavuzer D, Kefeli U, Tan A, Ercelep O, et al. Prognostic significance of ADAM17 expression in patients with gastric cancer who underwent curative gastrectomy. Clin Transl Oncol: Off Publ Fed Span Oncol Soc Nal Cancer Inst Mex. 2015;17(8):604–11. doi:10.1007/s12094-015-1283-1.
Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, Lopez M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005;280(20):19543–50. doi:10.1074/jbc.M410943200.
Aupperlee MD, Leipprandt JR, Bennett JM, Schwartz RC, Haslam SZ. Amphiregulin mediates progesterone-induced mammary ductal development during puberty. Breast Cancer Res: BCR. 2013;15(3):R44. doi:10.1186/bcr3431.
Davies KJ. The complex interaction of matrix metalloproteinases in the migration of cancer cells through breast tissue stroma. International Journal of Breast Cancer. 2014;2014:839094. doi:10.1155/2014/839094.
Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132(17):3923–33. doi:10.1242/dev.01966.
Glunde K, Stasinopoulos I. ADAM17: the new face of breast cancer-promoting metalloprotease activity. Cancer Biol Ther. 2009;8(11):1055–7.
Kenny PA. TACE: a new target in epidermal growth factor receptor dependent tumors. Differ: Res Biol Divers. 2007;75(9):800–8. doi:10.1111/j.1432-0436.2007.00198.x.
Kenny PA. Three-dimensional extracellular matrix culture models of EGFR signalling and drug response. Biochem Soc Trans. 2007;35(Pt 4):665–8. doi:10.1042/BST0350665.
Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27.
Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, et al. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62(12):3335–9.
Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J Clin Invest. 2007;117(2):337–45. doi:10.1172/JCI29518.
Al-Salihi MA, Ulmer SC, Doan T, Nelson CD, Crotty T, Prescott SM, et al. Cyclooxygenase-2 transactivates the epidermal growth factor receptor through specific E-prostanoid receptors and tumor necrosis factor-alpha converting enzyme. Cell Signal. 2007;19(9):1956–63. doi:10.1016/j.cellsig.2007.05.003.
Oshima H, Popivanova BK, Oguma K, Kong D, Ishikawa TO, Oshima M. Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Sci. 2011;102(4):713–9. doi:10.1111/j.1349-7006.2011.01847.x.
Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4(6):431–6.
Normanno N, Bianco C, De Luca A, Maiello MR, Salomon DS. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr-Relat Cancer. 2003;10(1):1–21.
Borrell-Pages M, Rojo F, Albanell J, Baselga J, Arribas J. TACE is required for the activation of the EGFR by TGF-alpha in tumors. EMBO J. 2003;22(5):1114–24. doi:10.1093/emboj/cdg111.
Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–98. doi:10.1093/jnci/djq366.
Jendraschak E, Sage EH. Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol. 1996;7(3):139–46.
Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784(1):150–8. doi:10.1016/j.bbapap.2007.09.008.
Romano M, De Francesco F, Gringeri E, Giordano A, Ferraro GA, Di Domenico M, et al. Tumor microenvironment versus cancer stem cells in cholangiocarcinoma: synergistic effects? J Cell Physiol. 2015. doi:10.1002/jcp.25190.
Whipple CA, Young AL, Korc M. A KrasG12D-driven genetic mouse model of pancreatic cancer requires glypican-1 for efficient proliferation and angiogenesis. Oncogene. 2012;31(20):2535–44. doi:10.1038/onc.2011.430.
Rego SL, Helms RS, Dreau D. Breast tumor cell TACE-shed MCSF promotes pro-angiogenic macrophages through NF-kappaB signaling. Angiogenesis. 2014;17(3):573–85. doi:10.1007/s10456-013-9405-2.
Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73(2):662–71. doi:10.1158/0008-5472.CAN-12-0653.
Curry JM, Eubank TD, Roberts RD, Wang Y, Pore N, Maity A, et al. M-CSF signals through the MAPK/ERK pathway via Sp1 to induce VEGF production and induces angiogenesis in vivo. PLoS One. 2008;3(10):e3405. doi:10.1371/journal.pone.0003405.
Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623–30. doi:10.1189/jlb.1107762.
Biswas SK, Lewis CE. NF-kappaB as a central regulator of macrophage function in tumors. J Leukoc Biol. 2010;88(5):877–84. doi:10.1189/jlb.0310153.
Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113(14):3139–46. doi:10.1182/blood-2008-12-172825.
Buchanan FG, Chang W, Sheng H, Shao J, Morrow JD, DuBois RN. Up-regulation of the enzymes involved in prostacyclin synthesis via Ras induces vascular endothelial growth factor. Gastroenterology. 2004;127(5):1391–400.
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66(23):11238–46. doi:10.1158/0008-5472.CAN-06-1278.
McDougall SR, Anderson AR, Chaplain MA. Mathematical modelling of dynamic adaptive tumour-induced angiogenesis: clinical implications and therapeutic targeting strategies. J Theor Biol. 2006;241(3):564–89. doi:10.1016/j.jtbi.2005.12.022.
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–5. doi:10.1038/nature10138.
Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25(16):2379–92. doi:10.1038/sj.onc.1209273.
Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, et al. Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci. 2007;98(5):674–84. doi:10.1111/j.1349-7006.2007.00440.x.
Yang J, Kim WJ, Jun HO, Lee EJ, Lee KW, Jeong JY, et al. Hypoxia-induced fibroblast growth factor 11 stimulates capillary-like endothelial tube formation. Oncol Rep. 2015;34(5):2745–51. doi:10.3892/or.2015.4223.
Li H, Chen J, Zen W, Xu X, Xu Y, Chen Q, et al. Effect of hypoxia inducible factor-1 antisense oligonucleotide on liver cancer. Int J Clin Exp Med. 2015;8(8):12650–5.
Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl 5):10–7. doi:10.1634/theoncologist.9-90005-10.
Xia C, Meng Q, Cao Z, Shi X, Jiang BH. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol. 2006;209(1):56–66. doi:10.1002/jcp.20707.
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology. 2005;7(2):134–53. doi:10.1215/S1152851704001115.
Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi:10.1016/S0065-230X(09)02002-8.
Wang XJ, Feng CW, Li M. ADAM17 mediates hypoxia-induced drug resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem. 2013;380(1–2):57–66. doi:10.1007/s11010-013-1657-z.
Hu L, Hofmann J, Jaffe RB. Phosphatidylinositol 3-kinase mediates angiogenesis and vascular permeability associated with ovarian carcinoma. Clin Cancer Res: An Off J Am Assoc Cancer Res. 2005;11(22):8208–12. doi:10.1158/1078-0432.CCR-05-0206.
Skinner HD, Zheng JZ, Fang J, Agani F, Jiang BH. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J Biol Chem. 2004;279(44):45643–51. doi:10.1074/jbc.M404097200.
Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277(15):12838–45. doi:10.1074/jbc.M112050200.
Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–4.
Tovey SM, Witton CJ, Bartlett JM, Stanton PD, Reeves JR, Cooke TG. Outcome and human epidermal growth factor receptor (HER) 1-4 status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res: BCR. 2004;6(3):R246–51. doi:10.1186/bcr783.
Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, et al. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66(12):6412–20. doi:10.1158/0008-5472.CAN-05-2368.
Vidal GA, Naresh A, Marrero L, Jones FE. Presenilin-dependent gamma-secretase processing regulates multiple ERBB4/HER4 activities. J Biol Chem. 2005;280(20):19777–83. doi:10.1074/jbc.M412457200.
Maatta JA, Sundvall M, Junttila TT, Peri L, Laine VJ, Isola J, et al. Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol Biol Cell. 2006;17(1):67–79. doi:10.1091/mbc.E05-05-0402.
Sartor CI, Zhou H, Kozlowska E, Guttridge K, Kawata E, Caskey L, et al. Her4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol Cell Biol. 2001;21(13):4265–75. doi:10.1128/MCB.21.13.4265-4275.2001.
Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–7. doi:10.1002/path.1370.
Kew TY, Bell JA, Pinder SE, Denley H, Srinivasan R, Gullick WJ, et al. c-erbB-4 protein expression in human breast cancer. Br J Cancer. 2000;82(6):1163–70. doi:10.1054/bjoc.1999.1057.
Cheng QC, Tikhomirov O, Zhou W, Carpenter G. Ectodomain cleavage of ErbB-4: characterization of the cleavage site and m80 fragment. J Biol Chem. 2003;278(40):38421–7. doi:10.1074/jbc.M302111200.
Ni CY, Yuan H, Carpenter G. Role of the ErbB-4 carboxyl terminus in gamma-secretase cleavage. J Biol Chem. 2003;278(7):4561–5. doi:10.1074/jbc.M210504200.
Kirkegaard T, Naresh A, Sabine VS, Tovey SM, Edwards J, Dunne B, et al. Expression of tumor necrosis factor alpha converting enzyme in endocrine cancers. Am J Clin Pathol. 2008;129(5):735–43. doi:10.1309/N6YB6YDVF58YCNHN.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111(10):1888–98. doi:10.1038/bjc.2014.388.
Duffy MJ, Crown J, Mullooly M. ADAM10 and ADAM17: new players in trastuzumab resistance. Oncotarget. 2014;5(22):10963–4.
Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney Jr DW, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421(6924):756–60. doi:10.1038/nature01392.
Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26(4 Suppl 12):78–83.
Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–40. doi:10.1016/j.ccr.2009.03.020.
Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28(6):803–14. doi:10.1038/onc.2008.432.
Gijsen M, King P, Perera T, Parker PJ, Harris AL, Larijani B, et al. HER2 phosphorylation is maintained by a PKB negative feedback loop in response to anti-HER2 herceptin in breast cancer. PLoS Biol. 2010;8(12):e1000563. doi:10.1371/journal.pbio.1000563.
Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28(18):5605–20. doi:10.1128/MCB.00787-08.
Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. doi:10.1038/nrm1962.
Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16(7):1647–55. doi:10.1093/emboj/16.7.1647.
Fridman JS, Caulder E, Hansbury M, Liu X, Yang G, Wang Q, et al. Selective inhibition of ADAM metalloproteases as a novel approach for modulating ErbB pathways in cancer. Clin Cancer Res: An Off J American Assoc Cancer Res. 2007;13(6):1892–902. doi:10.1158/1078-0432.CCR-06-2116.
Lendeckel U, Kohl J, Arndt M, Carl-McGrath S, Donat H, Rocken C. Increased expression of ADAM family members in human breast cancer and breast cancer cell lines. J Cancer Res Clin Oncol. 2005;131(1):41–8. doi:10.1007/s00432-004-0619-y.
Narita D, Seclaman E, Ursoniu S, Anghel A. Increased expression of ADAM12 and ADAM17 genes in laser-capture microdissected breast cancers and correlations with clinical and pathological characteristics. Acta Histochem. 2012;114(2):131–9. doi:10.1016/j.acthis.2011.03.009.
McGowan PM, Mullooly M, Caiazza F, Sukor S, Madden SF, Maguire AA, et al. ADAM-17: a novel therapeutic target for triple negative breast cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO. 2013;24(2):362–9. doi:10.1093/annonc/mds279.
Wang Y, Mo X, Piper MG, Wang H, Parinandi NL, Guttridge D, et al. M-CSF induces monocyte survival by activating NF-kappaB p65 phosphorylation at Ser276 via protein kinase C. PLoS One. 2011;6(12):e28081. doi:10.1371/journal.pone.0028081.
Lin EY, Pollard JW. Macrophages: modulators of breast cancer progression. Novartis Found Symp. 2004;256:158–68 .discussion 68-72, 259-69
Kelly PM, Davison RS, Bliss E, McGee JO. Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer. 1988;57(2):174–7.
Kawahara R, Lima RN, Domingues RR, Pauletti BA, Meirelles GV, Assis M, et al. Deciphering the role of the ADAM17-dependent secretome in cell signaling. J Proteome Res. 2014;13(4):2080–93. doi:10.1021/pr401224u.
Blaydon DC, Biancheri P, Di WL, Plagnol V, Cabral RM, Brooke MA, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med. 2011;365(16):1502–8. doi:10.1056/NEJMoa1100721.
Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, et al. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207(8):1617–24. doi:10.1084/jem.20092366.
Wada H, Saito K, Kanda T, Kobayashi I, Fujii H, Fujigaki S, et al. Tumor necrosis factor-alpha (TNF-alpha) plays a protective role in acute viral myocarditis in mice: a study using mice lacking TNF-alpha. Circulation. 2001;103(5):743–9.
Saftig P, Reiss K. The “a disintegrin and metalloproteases” ADAM10 and ADAM17: novel drug targets with therapeutic potential? Eur J Cell Biol. 2011;90(6–7):527–35. doi:10.1016/j.ejcb.2010.11.005.
Acknowledgments
We thank Liangpeng Li, M.D., for his discussion and help in revision. This study was funded by the National Natural Science Foundation of China (grant no. 81272470).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None
Additional information
Hongyu Shen and Liangpeng Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shen, H., Li, L., Zhou, S. et al. The role of ADAM17 in tumorigenesis and progression of breast cancer. Tumor Biol. 37, 15359–15370 (2016). https://doi.org/10.1007/s13277-016-5418-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5418-y